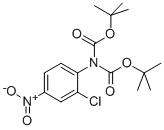
N,N-DIBOC-2-CHLORO-4-NITROANILINE synthesis
- Product Name:N,N-DIBOC-2-CHLORO-4-NITROANILINE
- CAS Number:252019-65-1
- Molecular formula:C16H21ClN2O6
- Molecular Weight:372.8

24424-99-5
821 suppliers
$13.50/25G

121-87-9
6 suppliers
$41.00/25g

252019-65-1
15 suppliers
$378.00/1g
Yield:252019-65-1 100%
Reaction Conditions:
with dmap in tetrahydrofuran at 20;
Steps:
Intermediate 75a: tert-butyl N-[(tert-butoxy)carbonyl]-N-(2-ch Ioro-4- nitrophenyl)carbamate
[00697] Intermediate 75a: tert-butyl N-[(tert-butoxy)carbonyl]-N-(2-ch Ioro-4- nitrophenyl)carbamate[00698] Di-tert-butyl dicarbonate (22.9g, 104.g3mmol) was added to a stirred solution of 2-chloro-4-nitro-aniline (8.63g, 50.01 mmol) and 4-dimethylamino pyridine, DMAP (0.3g, 2.46mmol) in THF (5OmL). The resulting solution was stirred over the weekend at room temperature. The solution was then concentrated in vacuo, and the residue dissolved in EtOAc (1 OOmL) and washed with 1 M citric acid solution (1 OOmL). The aqueous was extracted with further EtOAc (2 x 5OmL), and thecombined organic layers washed with brine (lOOmL), dried over Na2SO4 and concentrated in vacuoto give tert-butyl N-[(tert-butoxy)carbonyl]-N-(2-chloro-4-nitrophenyl)carbamate (1 8.5g, 50.01 mmol,100% yield) as an off-white solid which was used in the next step without further purification.1H NMR (CDCI3,400MHZ) O/ppm: 8.34 (1H, d, J= 2.4Hz), 8.16 (1H, dd, J= 8.6Hz, 2.4Hz), 7.42(1H, d, J= 8.6Hz), 1.42 (18H, 5).MS Method 2: RT: 2.06 mi mlz = 436 [M+Na+MeCN], 394 [M+Na]
References:
WO2016/51193,2016,A1 Location in patent:Paragraph 00696; 00697; 00698

1122-58-3
1047 suppliers
$6.00/1g

24424-99-5
821 suppliers
$13.50/25G

121-87-9
6 suppliers
$41.00/25g

252019-65-1
15 suppliers
$378.00/1g