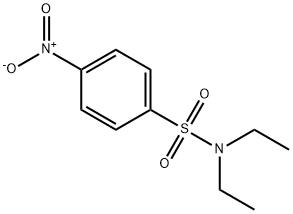
N,N-Diethyl-4-nitrobenzenesulfonaMide synthesis
- Product Name:N,N-Diethyl-4-nitrobenzenesulfonaMide
- CAS Number:89840-82-4
- Molecular formula:C10H14N2O4S
- Molecular Weight:258.29

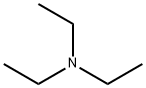
109-89-7
450 suppliers
$10.00/5g
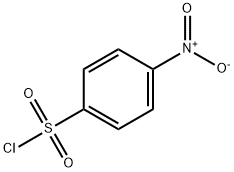
98-74-8
153 suppliers
$10.00/5g

89840-82-4
18 suppliers
$134.00/5mg
Yield:89840-82-4 69%
Reaction Conditions:
in dichloromethane; for 1 h;
Steps:
N,N-diethyl-4-nitro-benzenesulfonamide
To a stirring solution of 4-nitro-benzenesulfonyl chloride (10.0 g, 45.1 mmol) in CH2Cl2 (20 mL) was added NHEt2 (16.5 g, 226 mmol) and the reaction mixture stirred for 1 h. The reaction mixture was then dissolved in CH2Cl2 (200 mL) and washed with 2 M HCl (2 × 50 mL), brine (2 × 50 mL) and the organic fractions dried over MgSO4, concentrated in vacuo and re-crystallised in ethanol to give the product as an off-white solid (8.03 g, 69%); Rf = 0.55 (1:2 PE/EtOAc); m.p. 134-135 °C (EtOH); dH (600 MHz, CDCl3); 8.35 (d, J = 8.9 Hz, 2 H), 7.99 (d, J = 8.9 Hz, 2 H), 3.29 (q, J = 7.2 Hz, 4 H), 1.15 (t, J = 7.2 Hz, 6 H); dC (150 MHz, CDCl3) 149.9, 146.5, 128.2, 124.5, 42.3, 14.3. Data in agreement with that reported.[2],[3]
References:
Gray, Vincent James;Wilden, Jonathan D. [Tetrahedron Letters,2012,vol. 53,# 1,p. 41 - 44] Location in patent:supporting information; experimental part