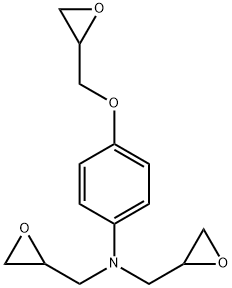
N,N-DIGLYCIDYL-4-GLYCIDYLOXYANILINE synthesis
- Product Name:N,N-DIGLYCIDYL-4-GLYCIDYLOXYANILINE
- CAS Number:5026-74-4
- Molecular formula:C15H19NO4
- Molecular Weight:277.32
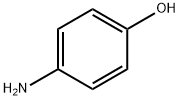
123-30-8
621 suppliers
$10.00/5g
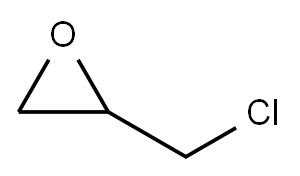
106-89-8
778 suppliers
$9.00/10 g

5026-74-4
190 suppliers
$37.39/50ml
Yield:5026-74-4 95%
Reaction Conditions:
Stage #1:4-amino-phenol;epichlorohydrin in water at 40; for 5 h;Inert atmosphere;Green chemistry;
Stage #2: with sodium hydroxide in water at 55; under 135.014 Torr;Green chemistry;Solvent;Temperature;Pressure;
Steps:
1 Example 1
Epichlorohydrin (507.3 g, 5.48 moles), water (42.35 g, 2.35 moles,) and p-aminophenol (60.5 g, 0.6 mole) were added in l-L 4 neck flask with condenser and a stirrer. Thetemperature of the reaction mixture was elevated to 40 °C, under the nitrogen atmosphere. The reaction temperature was maintained at 40 °C for 5 hours, to complete halohydrin adduct formation.Subsequently, the temperature of halohydrin adduct was raised to 60 °C followed by50% w/w aqueous sodium hydroxide solution (119.7 g) at a constant flow rate in 3-4180 mbar pressure, the water was removed with epichiorohydrin from the reactionazeotropically.To isolate crude halohydrin adduct, unreacted epichiorohydrin was recovered byat 90°C120 mbar.Water (262.5 g, 14.6 moles) was added to the crude triglycidyl aminophenol toalkali salt produced during cyclization reaction. Toluene (163.2 g, 1.7 moles) wasfor dissolving crude triglycidylaminophenol followed by the phase separation. Wastecontaining sodium chloride was removed in order to isolate the organic layer.The organic layer was subjected to distillation under reduced pressure (90 °C/20 mbar).product was refmed to suitable hydrolysable halogen content to get product with 99.7%0.05% epichlorohydrin content. The triglycidyl aminophenol was filtered, resulting in aviscosity at 25 °C of 35 Ps, 108.5 geq and hydrolysable halogen content 840 ppm.
References:
ADITYA BIRLA CHEMICALS (THAILAND) LTD.;DUBEY PRADIP, Kumar;KHULLAR, Alok;NAYAWAT, Thipa;VISATSINGH, Kamonsun;SAMUTHSEN, Patcharin;SAMANT, Prashant WO2015/174936, 2015, A1 Location in patent:Page/Page column 16