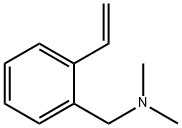
(N,N-DiMethylaMinoMethyl)styrene synthesis
- Product Name:(N,N-DiMethylaMinoMethyl)styrene
- CAS Number:22826-55-7
- Molecular formula:C11H15N
- Molecular Weight:161.24
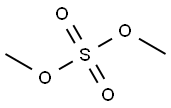
Yield:40398-01-4 10.92 g
Reaction Conditions:
Stage #1: 2-methyl-1,2,3,4-tetrahydroisoquinoline;dimethyl sulfate at 110; for 1.5 h;
Stage #2: with sodium hydroxide in isopropyl alcohol at 85; for 1 h;Hofmann Elimination;
Steps:
Synthesis of 2-(N,N-dialkyl)aminomethylstyrenes 4.
General procedure: A mixture of isoquinoline 10.0g (0.078 mol) and the corresponding alkyl halide or dialkyl sulfate (R1X = BnCl,Me2SO4, Et2SO4, 0.078 mol) was heated in a two-necked round-bottomed flask at110 °C under continuous stirring for 1.5 h. After that, formic acid 12.0 mL (14.8 g,0.326 mol) and triethylamine 12.0 mL (8.6 g, 0.086 mol) were added to the reactionmixture cooled to rt Then stirring and heating at 110 °C were continued for another 4-6 h until gas evolution ceased (a bubble counter was used). After cooling of thereaction mixture to rt, ≈60 mL of 20% NaOH solution (14.0 g, 0.32 mol of NaOH in 56mL of H2O) was added. The solution was stirred at rt for 15 min, diluted with Et2O (50mL), and stirred for another 15 min. The organic layer was separated and the waterphase was extracted with Et2O (50 mL). The combined organic phases were washedwith water (2 × 50 mL), dried over MgSO4 and filtered. The target N-alkyl-1,2,3,4-tetrahydroisoquinolines 3 were obtained as viscous, dark-brown oils after evaporation of the solvents under reduced pressure. These crude products weredirected to the next alkylation stage without further purification assuming 100% yield.A mixture of N-alkyl-1,2,3,4-tetrahydroisoquinoline (3, ≈0.078 mol) and thecorresponding alkyl halide or dialkyl sulfate (R2X, see Table 1, 0.078 mol) washeated at 110 °C and continuous stirring for 1.5 h in a two-necked round-bottomedflask. After cooling of the reaction mixture to rt, iPrOH (40 mL) and NaOH 6.40 g(0.16 mol) were added, then stirring and heating at 85 °C were continued for anotherhour. The solvent was evaporated under reduced pressure, then a mixture of H2O(60 mL) and Et2O (80 mL) was added, and the reaction mixture was stirred foradditional 30 min at rt. The organic layer was separated and the water phase was extracted with Et2O (80 mL). The combined organic phases were filtered throughsilica gel (4 × 3 cm), the sorbent was washed with Et2O (2 × 50 mL).After evaporation of the solvent under reduced pressure, crude 2-(N,Ndialkyl)aminomethylstyrenes (4) were obtained as viscous brown or dark-yellow oils.The analytical samples for analysis and catalysts synthesis were obtained afterpurification of the crude products by silica gel column chromatography using hexaneas an eluent, which gives target styrenes 4 as slightly yellow or colorless, viscousoils, in 60-88 % yields after four steps, see Table 1.
References:
Polyanskii, Kirill B.;Alekseeva, Kseniia A.;Raspertov, Pavel V.;Kumandin, Pavel A.;Nikitina, Eugeniya V.;Gurbanov, Atash V.;Zubkov, Fedor I. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 769 - 779] Location in patent:supporting information

1637-45-2
1 suppliers
inquiry
40398-01-4
48 suppliers
$19.00/1g

1612-65-3
34 suppliers
inquiry

40398-01-4
48 suppliers
$19.00/1g
83781-69-5
0 suppliers
inquiry

40398-01-4
48 suppliers
$19.00/1g
![Isoquinolinium, 1,2,3,4-tetrahydro-2-methyl-2-[(trimethylsilyl)methyl]-, iodide (1:1)](/CAS/20211123/GIF/134753-82-5.gif)
134753-82-5
0 suppliers
inquiry

40398-01-4
48 suppliers
$19.00/1g

1637-45-2
1 suppliers
inquiry
![Benzenemethanamine, 2-ethenyl-N-methyl-N-[(trimethylsilyl)methyl]-](/CAS/20210305/GIF/144437-09-2.gif)
144437-09-2
0 suppliers
inquiry
![Isoquinoline, 1,2,3,4-tetrahydro-2-methyl-1-[(trimethylsilyl)methyl]-](/CAS/20210305/GIF/144437-05-8.gif)
144437-05-8
0 suppliers
inquiry