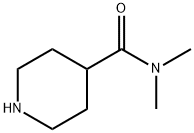
N,N-DIMETHYLPIPERIDINE-4-CARBOXAMIDE synthesis
- Product Name:N,N-DIMETHYLPIPERIDINE-4-CARBOXAMIDE
- CAS Number:1903-68-0
- Molecular formula:C8H16N2O
- Molecular Weight:156.23
![1-Piperidinecarboxylic acid, 4-[(dimethylamino)carbonyl]-, phenylmethyl ester](/CAS/20210305/GIF/887757-52-0.gif)
887757-52-0
2 suppliers
inquiry

1903-68-0
30 suppliers
$49.00/200μmol
Yield:1903-68-0 530 mg
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol;ethyl acetate at 10 - 35; for 5 h;
Steps:
1.C C) N,N-dimethylpiperidine-4-carboxamide
C)
N,N-dimethylpiperidine-4-carboxamide
Benzyl 4-(dimethylcarbamoyl)piperidine-1-carboxylate (960 mg) was dissolved in ethanol (15 mL) and ethyl acetate (15 mL), 10% palladium carbon (96 mg) was added thereto, and the mixture was stirred at room temperature for 5 hr under hydrogen atmosphere.
The insoluble substance was removed by filtration, and the filtrate was concentrated under reduced pressure to give the title compound (530 mg).
1H NMR (300 MHz, DMSO-d6) δ 1.31-1.57 (4H, m), 2.45 (1H, d, J = 3.0 Hz), 2.63 (1H, tt, J = 11.1, 4.0 Hz), 2.79 (3H, s), 2.91 (2H, dt, J = 12.1, 3.0 Hz), 2.99 (3H, s), 3.29 (2H, brs).
References:
EP2933247,2015,A1 Location in patent:Paragraph 0303

254905-58-3
30 suppliers
$423.72/1g

1903-68-0
30 suppliers
$49.00/200μmol
84358-13-4
387 suppliers
$5.00/5g

1903-68-0
30 suppliers
$49.00/200μmol
![1-[(Benzyloxy)carbonyl]piperidine-4-carboxylic acid](/CAS/GIF/10314-98-4.gif)
10314-98-4
264 suppliers
$6.00/1g

1903-68-0
30 suppliers
$49.00/200μmol