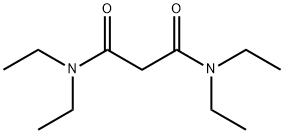
N,N,N',N'-TETRAETHYLMALONAMIDE synthesis
- Product Name:N,N,N',N'-TETRAETHYLMALONAMIDE
- CAS Number:33931-42-9
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3

141-82-2
739 suppliers
$5.00/25g

109-89-7
451 suppliers
$10.00/5g

33931-42-9
45 suppliers
$12.00/250mg
Yield: 86%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in chloroform at 20; for 18 h;
Steps:
2.2 Ligand Synthesis
General procedure: TEMA and TEDGA syntheses were carried out and supplied by Reading University,UK. TEMA was synthesized by the addition of 2 mol. equivalents of hydroxybenzotriazole(HOBt), triethylamine, diethylamine and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimidehydrochloride (EDC·HCl) to a suspension of malonic acid in chloroform. TEDGA wassynthesized by the addition of 2 mol. equivalents of HOBt, triethylamine, diethylamineand EDC·HCl to a suspension of 2,2′-oxydiacetic acid in chloroform. Both reactions werestirred at room temperature for 18 h. The crude reactions were washed with 1 mol·L-1 HCland 1 mol·L-1 NaOH. The combined organic layers were separately dried over MgSO4,filtered, and the solvent was removed under reduced pressure. The TEMA yield was86%. The TEDGA yield was 95%. TEMA 1H NMR (400 MHz, CDCl3)δ 3.46-3.37 (m,10H), 1.20 (t, J = 6.8 Hz, 6H), 1.14 (t, J = 7.2 Hz, 6H); 13C NMR (101 MHz, CDCl3)δ166.4, 42.6, 40.6, 40.3, 14.1, 12.8; (FTMS + pESI) calculated: C11H22N2Na[M+Na]+:237.1573 g·mol-1; observed: 237.1578 g·mol-1.
References:
Canner, Adam J.;Harwood, Laurence M.;Cowell, Joseph;Babra, Jasraj S.;Brown, Solomon F.;Ogden, Mark D. [Journal of Solution Chemistry,2020,vol. 49,# 1,p. 52 - 67]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

33931-42-9
45 suppliers
$12.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

109-89-7
451 suppliers
$10.00/5g

74-95-3
254 suppliers
$10.00/10g
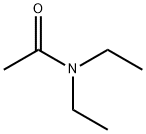
685-91-6
214 suppliers
$36.00/25mL
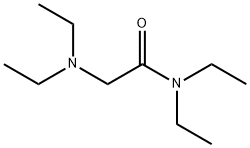
27794-54-3
17 suppliers
inquiry

33931-42-9
45 suppliers
$12.00/250mg

75-09-2
1199 suppliers
$10.00/25 mL
201230-82-2
1 suppliers
inquiry

109-89-7
451 suppliers
$10.00/5g

685-91-6
214 suppliers
$36.00/25mL

27794-54-3
17 suppliers
inquiry

33931-42-9
45 suppliers
$12.00/250mg
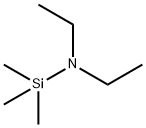
996-50-9
212 suppliers
$15.00/25g
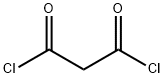
1663-67-8
215 suppliers
$36.79/5g

33931-42-9
45 suppliers
$12.00/250mg