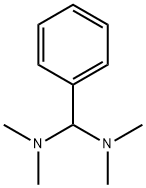
N,N,N,N-TETRAMETHYL-1-PHENYLMETHANEDIAMINE synthesis
- Product Name:N,N,N,N-TETRAMETHYL-1-PHENYLMETHANEDIAMINE
- CAS Number:13880-55-2
- Molecular formula:C11H18N2
- Molecular Weight:178.27

100-52-7
972 suppliers
$20.37/250g

124-40-3
545 suppliers
$18.00/100ml

13880-55-2
10 suppliers
inquiry
Yield:13880-55-2 17%
Reaction Conditions:
in water at 70; for 3 h;Sealed tube;
Steps:
3.1.5 Synthesis and hydrogenation of N,N-dimethylbenzylideneammonium chloride
Benzaldehyde (2.86 mL, 28.3 mmol, 1.00 equiv.) was placed in a 10 mL crimp seal glass vial filled with HNMe2 (40% in H2O) (6.00 mL, 53.2 mmol, 1.88 equiv.). The glass vial was sealed, and the two phased reaction mixture was heated under vigorous stirring up to 70 °C. After three hours, the reaction mixture was cooled to room temperature. The two-phased reaction mixture was then extracted twice with Et2O, and the combined organic phases were dried over Na2CO3. Evaporation of the volatiles yielded the crude product as a slight yellow solution. Purification was accomplished by vacuum distillation (0.2 mbar). After getting a fraction of benzaldehyde at room temperature, N,N,N’,N’-tetramethyl-1-phenylmethanediamine was obtained as a colorless liquid at ca. 30 °C .Yield: 17% (800 mg, 4.49 mmol). NMR spectroscopical data was in good agreement with the literature[SI11]. N,N,N’,N’-Tetramethyl-1-phenylmethanediamine (300 mg, 1.68 mmol, 1.00 equiv.) was placed in a SCHLENK flask and dissolved in Et2O (5 mL): The reaction mixture was cooled in an ice/water bath and acetyl chloride (0.160 mL, 1.68 mmol, 10.0 equiv.) was added dropwise under vigorous stirring. During the addition, a white precipitate was formed. After addition was completed, the reaction mixture was allowed to reach room temperature and stirred for an hour. The solvent was then decanted off, the white solid was washed three times with Et2O and dried under vacuum. Yield: 98% (280 mg, 1.65 mmol). NMR spectroscopical data was in good agreement with the literature[SI11]. In a glovebox B(2,6-F2-C6H3)3 (3c) (7.0 mg, 20 μmol, 20 mol%) and N,N-dimethylbenzylideneammonium chloride (2c) (17.0 mg, 100 μmol, 1.00 equiv.) were dissolved in 0.6 mL CDCl3 and transferred to a J. YOUNG NMR tube with Teflon tap. The sample was then frozen in liquid nitrogen, the headspace was evacuated, and the sample was charged with hydrogen at -196 °C. After sealingand thawing, the hydrogen pressure inside the sample reached approximately 4 bar. The sample was then heated to 70 °C on a shaking plate to ensure hydrogen exchange. After 18 h the NMR tube was cooled to room temperature and the crude reaction mixture was analyzed by 1H-NMR spectroscopy. Pure N,N-dimethylbenzylammonium chloride (4c·HCl) could be detected without traces of starting material or other degradation products. A sample without borane 3c showed noformation of ammonium salt 4c·HCl.
References:
K?ring, Laura;Sitte, Nikolai A.;Paradies, Jan [Synthesis,2022,vol. 54,# 5,p. 1287 - 1300] Location in patent:supporting information
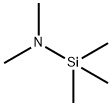
2083-91-2
149 suppliers
$30.76/10g

100-52-7
972 suppliers
$20.37/250g

107-46-0
471 suppliers
$10.00/10g

13880-55-2
10 suppliers
inquiry

2083-91-2
149 suppliers
$30.76/10g

100-52-7
972 suppliers
$20.37/250g

13880-55-2
10 suppliers
inquiry