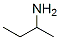
N-(SEC-BUTYL)-2-CHLOROACETAMIDE synthesis
- Product Name:N-(SEC-BUTYL)-2-CHLOROACETAMIDE
- CAS Number:32322-73-9
- Molecular formula:C6H12ClNO
- Molecular Weight:149.62
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 28; for 2 h;
Steps:
77.77A Example 77A
[0345] To the mixture of 2-methylpropan-l -amine (1.94 g, 26.57 mmol, 2.70 mL) and TEA (5.38 g, 53.12 mmol, 7.37 mL) in CH2CI2 (30.00 mL) was added dropwise 2-chloroacetyl chloride (2.00 g, 17.71 mmol, 1.41 mL) at 0 °C. The mixture was stirred at 28 °C for 2 h. TLC (petroleum ether/EtOAc=2: l, Rf =0.7) detected one new main spot. The mixture was diluted with CH2CI2 (30 mL) and washed with water (40 mLx2), citric acid (10%, 40 mLx2), NaHC03 (40 mLx2), brine (40 mLx2). The organic layer was dried over Na2S04, filtered and concentrated under reduced pressure to give the title compound (2.60 g, crude) as a black oil and used for the next step reaction without further purification. 1H NMR (400 MHz, DMSO-i/6) δ 8.00-7.98 (m, 1H), 4.00 (s, 2H), 3.71-3.61 (m, 1H), 1.43-1.36 (m, 2H), 1.03 (d, J= 6.8 Hz, 3H), 0.82 (t, J= 7.4 Hz, 3H).
References:
WO2016/210330,2016,A1 Location in patent:Paragraph 0344-0345
78-81-9
188 suppliers
$19.60/250ml

79-04-9
352 suppliers
$12.00/5g

32322-73-9
21 suppliers
$46.00/1g