
N-tert-butyl-3-nitropyridin-2-amine synthesis
- Product Name:N-tert-butyl-3-nitropyridin-2-amine
- CAS Number:79371-45-2
- Molecular formula:C9H13N3O2
- Molecular Weight:195.22
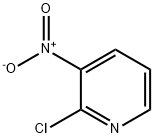
5470-18-8
433 suppliers
$6.00/5g
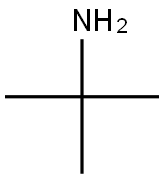
75-64-9
419 suppliers
$10.00/10g

79371-45-2
17 suppliers
$45.00/250mg
Yield:79371-45-2 73%
Reaction Conditions:
in 1-methyl-pyrrolidin-2-one at 60; for 5 h;
Steps:
16.1 [04891 Step 1: tert-Butyl-(3-nitro-pyridin-2-yl)-amine
[0490] To a solution of compound I (20 g, 127 mmol, 1 eq) in N-methyl-2-pyrrolidine (100 mL, 1.04 mol, 8.2 eq) was added tert-butyl amine (100 mL, 950 mmol, 7.5 eq) and the resulting mixture was stirred at 60 °C for 5 h. The mixture was poured into ice-cold iN aqueous HC1 solution (500 mL) and the organic components were extracted with ethyl acetate (3 x 300 mL). The combined organic layers were washed with saturated aqueous sodium bicarbonate solution (400 mL) and brine (2 x 300 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to provide crude compound. The crude product was purified by silica gel (100-200 mesh) column chromatography using 2% ethyl acetate/hexanes to obtain the title compound (18 g, 73 %) as yellow viscous liquid. 1H NMR (400 MHz, DMSO-d6) ? 8.48 (dd, 1H, J = 1, 4 Hz), 8.41 (dd, 1H, J = 2, 8 Hz), 8.20 (brs, 1H), 6.76 (dd, 1H, J = 4, 8 Hz), 1.51 (s, 9H).
References:
WO2015/95128,2015,A1 Location in patent:Paragraph 0488; 0489; 0490