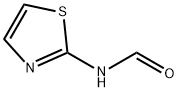
N-Thiazol-2-yl-forMaMide synthesis
- Product Name:N-Thiazol-2-yl-forMaMide
- CAS Number:25602-39-5
- Molecular formula:C4H4N2OS
- Molecular Weight:128.15

96-50-4
513 suppliers
$5.00/5g
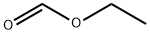
109-94-4
464 suppliers
$10.00/10g

25602-39-5
13 suppliers
$45.00/50mg
Yield:25602-39-5 88%
Reaction Conditions:
with N1-(3-(trimethoxysilyl)propyl)ethane-1,2-diamine-sulfamic acid supported on mesoporous silica SBA-15 in neat (no solvent) at 50;Green chemistry;
Steps:
General procedure for N-formylation
General procedure: To a mixture of aniline (1 mmol) and ethyl formate (1.5 mmol),1 mol% of SBA-15/PrEn-NHSO3H was added and the mixture was stirred at 50°C under solvent free condition. The progress of the reaction was monitored by TLC. After completion of the reaction,ethyl acetate was added to the solidified mixture and the solid catalyst was separated by filtration. The filtrate was dried over anhydrous Na2SO4. The solvent was evaporated and the product,obtained, was subjected to column chromatography. The isolated catalyst could be reused by addition of new portions of substrate.The structure of products obtained had been confirmed by physical properties such as 1H NMR. 1H NMR (300.13 MHz, CHCl3): 7.40(s,CHO), 7.74(d, J 8.7 Hz, H-Ar), 8.24(d, J 8.7 Hz, H-Ar), 8.49(NH).
References:
Rostamnia, Sadegh;Doustkhah, Esmail [Journal of Molecular Catalysis A: Chemical,2016,vol. 411,p. 317 - 324]

96-50-4
513 suppliers
$5.00/5g

64-18-6
1061 suppliers
$22.12/250ML

25602-39-5
13 suppliers
$45.00/50mg

96-50-4
513 suppliers
$5.00/5g
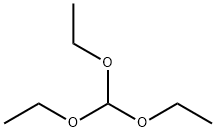
122-51-0
486 suppliers
$10.00/5ml

25602-39-5
13 suppliers
$45.00/50mg
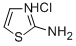
6142-05-8
114 suppliers
$10.00/1g

25602-39-5
13 suppliers
$45.00/50mg

96-50-4
513 suppliers
$5.00/5g
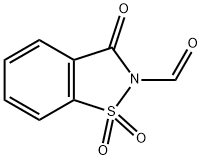
50978-45-5
97 suppliers
$35.00/1g

25602-39-5
13 suppliers
$45.00/50mg