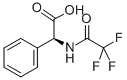
(+)-N-TRIFLUOROACETYL-L-PHENYLGLYCINE synthesis
- Product Name:(+)-N-TRIFLUOROACETYL-L-PHENYLGLYCINE
- CAS Number:155894-96-5
- Molecular formula:C10H8F3NO3
- Molecular Weight:247.17
Yield:155894-96-5 94%
Reaction Conditions:
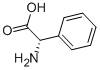
Stage #1: (S)-2-phenylglycinewith triethylamine in methanol; for 0.25 h;Cooling with ice;
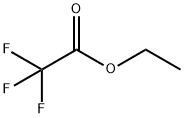
Stage #2: ethyl 2,2,2-trifluoroacetate in methanol at 20;
Steps:
2-Phenyl-2-(2,2,2-trifluoroacetamido)acetic acid (TFA-Phg-OH, 2)
General procedure: Triethylamine (8.30 mL, 59.5 mmol) was added to a suspension of optically pure phenylglycine (6.00 g,39.7 mmol) in MeOH (66 mL) on ice. After stirring for 15 min, ethyl trifluoroacetate (6.14 mL, 51.6mmol) was added. The suspension was turned to solution with stirring at rt for overnight. Afterconcentration, the residue was dissolved in H2O (100 mL) and acidified with concentrated HCl 4 mL,then extracted with EtOAc. The organic layer was washed with brine, dried by MgSO4, filtered, andconcentrated with oil pump to afford a colorless solid.L-2: 9.24 g (94%), [α]D +171 (c 1.0, MeOH), HRMS-ESI (m/z) [M+Na]+ calcd for C10H8F3NO3Na+270.0354, found 270.0368.
References:
Wang, Zeping;Ishikawa, Shoko;Ohashi, Fumina;Sagisaka, Reo;Murai, Yuta;Tachrim, Zetryana Puteri;Suzuki, Takeyuki;Hashimoto, Makoto [Heterocycles,2022,vol. 105,# 1,p. 406 - 416]