
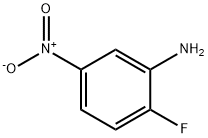
N1-(2-FLUORO-5-NITROPHENYL)ACETAMIDE synthesis
- Product Name:N1-(2-FLUORO-5-NITROPHENYL)ACETAMIDE
- CAS Number:454-07-9
- Molecular formula:C8H7FN2O3
- Molecular Weight:198.15
Yield:454-07-9 89%
Reaction Conditions:
with acetic acid for 0.666667 h;Reflux;
Steps:
7.A Step A
Commercially available 2-fluoro-5-nitrophenylamine (7.8 g, 50 mmol) was suspended in acetic acid (50 ml_) and heated to reflux. Then acetic acid anhydride (8 ml_) was added over a period of 10 minutes. After the addition was completed, the mixture was heated at reflux for another 30 minutes. The mixture was allowed to cool to ~40°C and poured into ice-water (800 ml_). The precipitate was collected by filtration, washed with water (50 ml_) and air-dried to afford the title compound as a beige solid (8.9 g, 89 %). 1H-NMR (400 MHz, CDCI3): δ = 2.27 (s, 3H), 7.23-7.26 (m, 1 H), 7.50 (br-s, 1 H), 7.97-8.01 (m, 1 H), 9.31 (s, 1 H)
References:
AC IMMUNE SA;PIRAMAL IMAGING SA;KROTH, Heiko;NAMPALLY, Sreenivasachary;MOLETTE, Jérôme;GABELLIERI, Emanuele;BENDERITTER, Pascal, André, René;FROESTL, Wolfgang;SCHIEFERSTEIN, Hanno;MUELLER, Andre;SCHMITT-WILLICH, Heribert;BERNDT, Mathias WO2015/110263, 2015, A1 Location in patent:Page/Page column 142
369-36-8
251 suppliers
$10.00/10g

454-07-9
15 suppliers
inquiry

369-36-8
251 suppliers
$10.00/10g

108-24-7
5 suppliers
$14.00/250ML

64-19-7
1535 suppliers
$10.00/25ML

454-07-9
15 suppliers
inquiry

70-34-8
11 suppliers
$10.00/5g

454-07-9
15 suppliers
inquiry
