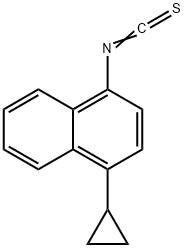
NAPHTHALENE, 1-CYCLOPROPYL-4-ISOTHIOCYANATO- synthesis
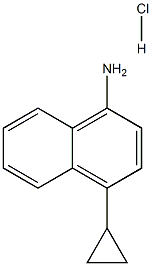
- Product Name:NAPHTHALENE, 1-CYCLOPROPYL-4-ISOTHIOCYANATO-
- CAS Number:878671-95-5
- Molecular formula:C14H11NS
- Molecular Weight:225.31

75-15-0
248 suppliers
$40.00/100 g

878671-94-4
90 suppliers
$45.00/250mg

878671-95-5
148 suppliers
$174.00/5g
Yield: 94%
Reaction Conditions:
Stage #1:carbon disulfide;1-amino-4-cyclopropylnaphthalene with potassium carbonate in water;N,N-dimethyl-formamide at 15; for 3.5 h;
Stage #2: with 1,3,5-trichloro-2,4,6-triazine in dichloromethane;water;N,N-dimethyl-formamide at 0; for 1 h;
Stage #3: with water;sodium hydroxide in dichloromethane;N,N-dimethyl-formamide for 2 h;
Steps:
2 Example 2. 4 -cyclopropyl-l-isothiocyanate naphthalene
Example 2. 4 -cyclopropyl-l-isothiocyanate naphthalene [067] To a flask added 4-cyclopropyl-l-naphthylamine prepared by example 1 (66 g), K2C03 (100 g), DMF (80 ml) and water (560 mL), the mixture was stirred at room temperature, then CS2 (44 mL) was drop wise added to the mixture in 5 min. After the addition was complete, the mixture was stirred for another 3.5 hours at room temperature (15°C). Then, the mixture was cooled to 0°C, and a solution of cyanuric chloride (33.6 g) in 250 mL of CH2C12 was drop wise added. After the addition was complete, the mixture was stirred for another 1 hour to complete the conversion. The resulting mixture was added 6 N NaOH (300 mL) and was stirred for another 2 hours. To the resulting mixture added dichloromethane (400 mL) and water (200 mL), then the organic layer was separated and the aqueous layer was extracted with CH2C12 (250 mL><2). The combined organic layers were dried over anhydrous Na2S04, filtered, and the solvent of filtrate was removed via distillation under atmospheric pressure to obtain a brown liquid. The brown liquid was further purified by a short silica gel column (cyclohexane as eluent), then 77.0 g (94%) of colorless liquid 4 -cyclopropyl-l-isothiocyanate naphthalene was obtained. GC-Ms: m/z (EI): 225, 1H MR (400 MHz, CDC13): δ 8.43 (m, 1H), 8.12 (m, 1H), 7.62 (m, 2H), 7.33 (d, J=8.0 Hz, 1H), 7.18 (d, J=8.0 Hz, 1H), 2.31 (m, 1H), 1.10 (m, 2H), 0.77 (m, 2H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;CHEN, Weiqiang;LUO, Jian;LIU, Lixue;FAN, Yuping WO2014/198241, 2014, A1 Location in patent:Paragraph 066-067

878671-94-4
90 suppliers
$45.00/250mg
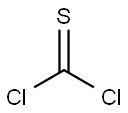
463-71-8
145 suppliers
$29.89/5g

878671-95-5
148 suppliers
$174.00/5g

878671-94-4
90 suppliers
$45.00/250mg
6160-65-2
408 suppliers
$6.00/1g

878671-95-5
148 suppliers
$174.00/5g

878671-94-4
90 suppliers
$45.00/250mg
540-72-7
433 suppliers
$13.50/100G

878671-95-5
148 suppliers
$174.00/5g

463-71-8
145 suppliers
$29.89/5g
1533519-92-4
151 suppliers
$6.00/250mg

878671-95-5
148 suppliers
$174.00/5g