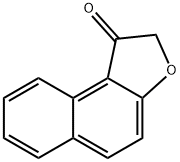
Naphtho[2,1-b]furan-1-one synthesis
- Product Name:Naphtho[2,1-b]furan-1-one
- CAS Number:19997-42-3
- Molecular formula:C12H8O2
- Molecular Weight:184.19
Yield:19997-42-3 97%
Reaction Conditions:
aluminium chloride in toluene;
Steps:
3 N-{1-(1,2-Dihydronaphtho[2,1-b]furyl}-N-hydroxyurea
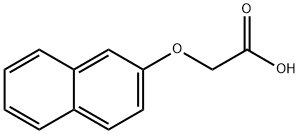
1,2-Dihydronaphtho[2,1-b]furan-1-one. A mixture of 2-(2-naphthoxy)acetic acid (3.06 g, 15.1 mmol) and oxalyl chloride (7 mL, 80.3 mmol) was heated at reflux for 45 min, then allowed to cool and concentrated under reduced pressure. The residue was dissolved in toluene, and to this was added aluminium chloride (2.40 g, 18.1 mmol). The resulting mixture was stirred at room temperature for 1 h, then poured into ice. The organic phase was separated and washed with saturated aqueous NaCl. Removal of the solvent in vacuo provided the title compound (2.71 g, 97%). 1 H NMR (CDCl3 /MeOH-d4): δ8.74 (d, 1H); 8.06 (d, 1H); 7.82 (d, 1H); 7.66 (ddd, 1H); 7.50 (ddd, 1H); 7.25 (d, 1H); 4.54 (s, 2H).
References:
US5140047,1992,A
120-23-0
327 suppliers
$7.00/25g
![Naphtho[2,1-b]furan-1-one](/CAS/GIF/19997-42-3.gif)
19997-42-3
8 suppliers
inquiry

40926-77-0
14 suppliers
$80.00/100mg
![Naphtho[2,1-b]furan-1-one](/CAS/GIF/19997-42-3.gif)
19997-42-3
8 suppliers
inquiry
708-06-5
286 suppliers
$14.00/5g
![Naphtho[2,1-b]furan-1-one](/CAS/GIF/19997-42-3.gif)
19997-42-3
8 suppliers
inquiry