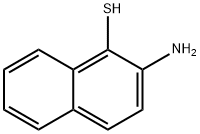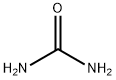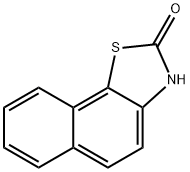
Naphtho[2,1-d]thiazol-2(3H)-one (9CI) synthesis
- Product Name:Naphtho[2,1-d]thiazol-2(3H)-one (9CI)
- CAS Number:17931-24-7
- Molecular formula:C11H7NOS
- Molecular Weight:201.24

124-38-9
134 suppliers
$214.00/14L
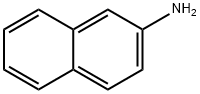
91-59-8
212 suppliers
$61.39/100mg
![Naphtho[2,1-d]thiazol-2(3H)-one (9CI)](/CAS/GIF/17931-24-7.gif)
17931-24-7
5 suppliers
inquiry
Yield:17931-24-7 91%
Reaction Conditions:
with potassium tert-butylate;sulfur in acetonitrile at 70; under 11251.1 Torr; for 8 h;Autoclave;
Steps:
2.6. The global method for synthesis of thiazolidin-2-one
General procedure: Aniline (1.5 mmol), S8 (1.5 mmol), AuCellulose/DFNS NPs (8 mg),CH3CN (10 mL) and tBuOK (4 eq.) were added to a 150 mL autoclave.The closed autoclave was sanitized twice with CO2 gas, pressurized withCO2 (1.5 MPa), and warmed below 70 C for 8 h. In the next step, thereaction mixture was unheated to ambient temperature, the remainingCO2 gas was removed, and the reactor was opened. The reaction wascontrolled by TLC. After completion, ethanol was added to the reactionmixture and it was filtered to separate the catalyst. Next, the solvent wasremoved from the solution under dropped pressure and the outcome wascleansed by recrystallization deploying ethyl acetate/n-hexane.
References:
Wei, Xingyue;Wang, Xingmin [Inorganic Chemistry Communications,2021,vol. 128,art. no. 108590]

124-38-9
134 suppliers
$214.00/14L
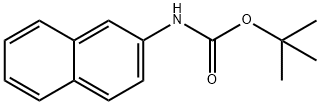
454713-45-2
16 suppliers
$130.00/2.5g
![Naphtho[2,1-d]thiazol-2(3H)-one (9CI)](/CAS/GIF/17931-24-7.gif)
17931-24-7
5 suppliers
inquiry
![Naphtho[2,1-d]thiazole, 2-chloro- (7CI,8CI,9CI)](/CAS/GIF/17931-25-8.gif)
17931-25-8
7 suppliers
inquiry
![Naphtho[2,1-d]thiazol-2(3H)-one (9CI)](/CAS/GIF/17931-24-7.gif)
17931-24-7
5 suppliers
inquiry