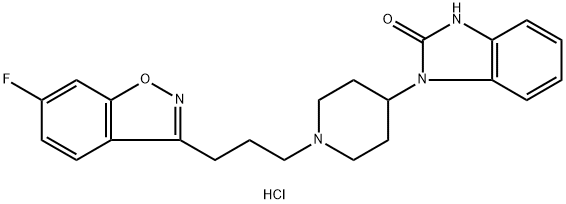
Neflumozide hydrochloride synthesis
- Product Name:Neflumozide hydrochloride
- CAS Number:86015-38-5
- Molecular formula:C22H24ClFN4O2
- Molecular Weight:430.91
Yield:86015-38-5 2.7 g (52%)
Reaction Conditions:
with sodium hydrogencarbonate in N-methyl-acetamide;water;
Steps:
{1-[3-(6-Fluoro-1,2-benzisoxazol-3-yl)propyl]-4-(2-oxo-1-benzimidazolinyl)}piperidine hydrochloride
{1-[3-(6-Fluoro-1,2-benzisoxazol-3-yl)propyl]-4-(2-oxo-1-benzimidazolinyl)}piperidine hydrochloride To 25 ml of dry dimethylformamide was added 2.6 g of 4-(2-oxo-1-benzimidazolinyl)piperidine, 2.78 g of 3-(3-chloropropyl)-6-fluoro-1,2-benzisoxazole, 8.0 g of sodium bicarbonate, and a crystal of potassium iodide. After stirring at 100° C. for one hr, the mixture was cooled, filtered, and the dimethylformamide solution evaporated to an oil. The oil was stirred with 100 ml of water for five mins and extracted with ether/ethyl acetate. The organic layer was washed with water (2*), saturated sodium chloride solution and dried over anhydrous magnesium sulfate. After filtering, the solvents were evaporated to an oil, which was dissolved in ether, and converted to a salt by treatment with ethereal hydrogen chloride. Recrystallization from ethyl acetate/methanol (5:1) gave 2.7 g (52%) of product, mp 255°-257° C. Analysis: Calculated for C22 H23 FN4 O2 HCl: 61.32% C; 5.61% H; 13.00% N. Found: 61.14% C; 5.68% H; 12.88% N.
References:
US4390544,1983,A
![1-Chloro-3-(6-fluoro-benzo[d]isoxazol-3-yl)-propane](/CAS/20180528/GIF/84243-02-7.gif)