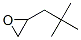
Neopentyloxirane synthesis
- Product Name:Neopentyloxirane
- CAS Number:2245-29-6
- Molecular formula:
- Molecular Weight:0

762-62-9
59 suppliers
$60.00/500mg
2245-29-6
0 suppliers
inquiry
Yield:2245-29-6 82%
Reaction Conditions:
Stage #1: 4,4-dimethylpent-1-enewith pyridine-3-carbonitrile;dihydrogen peroxide;methyltrioxorhenium(VII) in dichloromethane;water at 20 - 25;
Stage #2: with manganese(IV) oxide in dichloromethane;
Steps:
2.A
A solution of 4, 4-dimethyl- 1-PENTENE (8.05 g, 82 mmol) in CH2C12 (45 mL) was treated with METHYLTRIOXORHENIUM (0.10 g, 0.41 mmol) and put into a water bath and stirring was initiated. The reaction mixture was treated with 3-cyanopyridine (853 mg) and stirred until the material dissolved. A solution of 31 % aqueous hydrogen peroxide (11.7 mL, 107 mmol) was then added dropwise over the course of about 30 minutes, keeping the temperature at 20 to 25 °C. The reaction was allowed to stir overnight. The contents of the flask were then transferred to a seperatory FUNNEL. A separate flask was charged with 15 mg OF MN02 and a stir bar and cooled with an ice bath. The organic layer was slowly poured into this flask, allowing ample time for the hydrogen peroxide to be quenched. After 15 minutes of stirring, sodium sulfate (-20 g) was added and stirring was continued for an additional 15 minutes. The solution was then transferred to a 100 mL pear shaped flask. The CH2CL2 was distilled off at atmospheric pressure through a Vigreaux column. After the initial distillation, the Vigreaux was removed and replaced with a regular short path distillation head. The flask was heated to 120°C and a mild vacuum was applied to transfer over 7.7 g (82%) of product as a colorless liquid
References:
WO2005/18568,2005,A2 Location in patent:Page/Page column 39