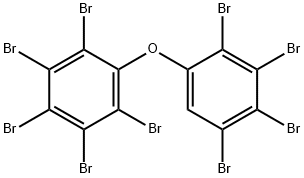
2,2',3,3',4,4',5,5',6-nonabromodiphenylether synthesis
- Product Name:2,2',3,3',4,4',5,5',6-nonabromodiphenylether
- CAS Number:63387-28-0
- Molecular formula:C12HBr9O
- Molecular Weight:880.27
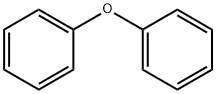
101-84-8
603 suppliers
$5.00/25g

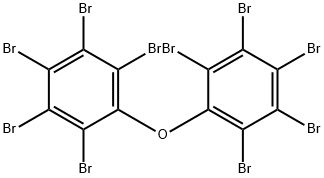
63387-28-0
33 suppliers
$479.00/1ml
1163-19-5
370 suppliers
$13.00/25g
Yield: 99.44 - 99.70 %Chromat. , 0.233 - 0.526 %Chromat.
Reaction Conditions:
Stage #1:diphenylether with bromine at 40;
Stage #2: with bromine;aluminum (III) chloride at 56.5 - 78.4; under 1385.82 - 1458.22 Torr; for 3.65 - 4.36667 h;Product distribution / selectivity;
Steps:
9; 10
EXAMPLE 9; Preparation of Partially brominated DPO[0062] A batch of partially brominated DPO was prepared by slowly adding 131 g of bromine to 236 g of DPO while cooling in a water bath so that the temperature did not exceed 400C. The resultant partially brominated DPO contained about 0.59 atom of bromine per molecule of DPO. Catalyzed Bromination[0063] In a 1 -liter jacketed reactor equipped with 00C Friedrich condenser, mechanical stirrer, thermocouple well, and 1/32-inch LD. diptube was placed 4.0 g AlCl3 and 1021 g of bromine. To this was fed 64.3 g of a portion of the above batch of partially brominated DPO.The feed rate was approximately 0.17 mL per minute with the condenser water set at +10C. The feed occurred over a period of 4 hours and 17 minutes during which time the reaction temperature was maintained at 56.5 to 56.90C. The mixture was refluxed for an additional period of about 5 minutes as the temperature rose to 590C and then 450 mL of water was added to it. Bromine was distilled off from the reaction mixture up to a temperature of ca800C. The amount of bromine collected was 502 g. Water was then decanted from the reaction mixture, and the remainder of the reaction mixture was stirred in 400 mL of water and this wash water was decanted. Then 500 mL of 2% NaOH was added. Product was collected and water washed. GC analysis showed 0.067% and 0.233% of the first and second nonabromodiphenyl oxide isomers, respectively, the second being the ortho isomer, and99.70% of decabromodiphenyl oxide. EXAMPLE 10Catalyzed Bromination[0064] Another portion of the same partially brominated DPO as used in Example 9 was used as the feed in this Example. The reactor was a 500 mL pressure reactor (Ace glassNo.6438-17) with jacket, equipped with a mechanical stirrer, a 1/32-inch diptube to feed the partially brominated diphenyl oxide, 1/4-inch O. D. diptube for takeoff, and a 9 inch x 3/4-inch LD. (ca. 22.8 cm x 1.91 cm) uninsulated fractionation column packed with 5 mm x 5 mm Raschig rings. The column was fitted at the top with a 00C condenser. The condenser was connected to a backpressure regulator to maintain the desire pressure. To this reactor were changed 3.9 g OfAlCl3 and 940 g of bromine. A 90 mL pressure bottle was charged with 59.8 g of the above partially brominated diphenyl oxide having about 0.6 bromine atom per molecule of diphenyl oxide. The pressure bottle was equalized with reactor via a nitrogen purge that entered the system at a back pressure regulator. The contents of the 500 mL pressure reactor were heated to a refluxing temperature of 78.4°C at a pressure of 12.1 psig(ca. 1.85x 105 Pa). Then a feed of the partially brominated DPO was maintained at a flow rate
References:
ALBEMARLE CORPORATION WO2008/27776, 2008, A2 Location in patent:Page/Page column 14-15

1163-19-5
370 suppliers
$13.00/25g
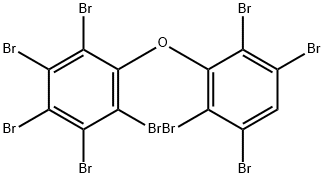
437701-78-5
15 suppliers
$185.00/10mg

63387-28-0
33 suppliers
$479.00/1ml
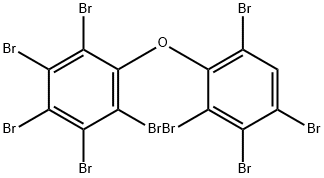
437701-79-6
20 suppliers
$389.00/1ml

101-84-8
603 suppliers
$5.00/25g

437701-78-5
15 suppliers
$185.00/10mg

63387-28-0
33 suppliers
$479.00/1ml

437701-79-6
20 suppliers
$389.00/1ml

1163-19-5
370 suppliers
$13.00/25g

101-84-8
603 suppliers
$5.00/25g

437701-78-5
15 suppliers
$185.00/10mg

63387-28-0
33 suppliers
$479.00/1ml

437701-79-6
20 suppliers
$389.00/1ml