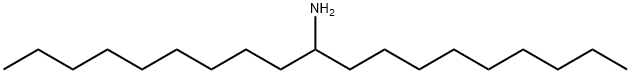
nonadecan-10-amine synthesis
- Product Name:nonadecan-10-amine
- CAS Number:3241-23-4
- Molecular formula:C19H41N
- Molecular Weight:283.54
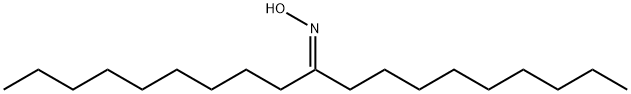
86325-38-4
0 suppliers
inquiry

3241-23-4
17 suppliers
inquiry
Yield:3241-23-4 408 mg
Reaction Conditions:
with sodium bis(2-methoxyethoxy)aluminium dihydride in toluene; for 2 h;Reflux;
Steps:
2.2.1 10-Nonadecanamine
The oxime was then dissolved in 10ml of dry toluene and 2ml of 70% sodium bis(2-methoxyethoxy)aluminium hydride (RedAl) in toluene were dropwise added. There is an important gas evolution as the reductive agent is added which requires a particular attention when the reaction is carried out. The reaction mixture was refluxed for 2h, then cooled to the room temperature and carefully added to 10ml of 5% HCl. At the end an additional quantity (3-4ml) of concentrated HCl was added in order to dissolve the aluminium salts. The solution was extracted with hexane, washed with 5% NaOH solution, water and then dried over MgSO4. After the evaporation of the solvent were obtained 408mg of 10-nonadecanamine (89%). 1H NMR (CDCl3): δ 0.81 (t, 6H), 1.20 (m, 32H), 2.14 (br, 2H), 2.68 (m, 1H). IR (KBr, cm-1): υ 3372, 3256, 2494, 1463, 1377, 801, 727.
References:
Kozma, Erika;Kotowski, Dariusz;Catellani, Marinella;Luzzati, Silvia;Famulari, Antonino;Bertini, Fabio [Dyes and Pigments,2013,vol. 99,# 2,p. 329 - 338]
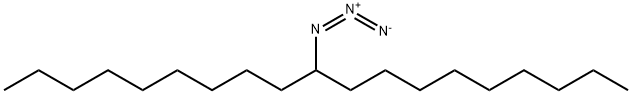
943545-46-8
0 suppliers
inquiry

3241-23-4
17 suppliers
inquiry
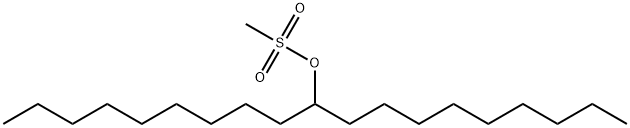
943545-41-3
0 suppliers
inquiry

3241-23-4
17 suppliers
inquiry
693-58-3
215 suppliers
$5.00/5g

3241-23-4
17 suppliers
inquiry
16840-84-9
38 suppliers
inquiry

3241-23-4
17 suppliers
inquiry