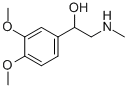
normacromerine synthesis
- Product Name:normacromerine
- CAS Number:5653-66-7
- Molecular formula:C11H17NO3
- Molecular Weight:211.26
Yield:5653-66-7 95.1%
Reaction Conditions:
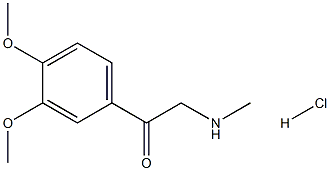
Stage #1: 1-(3,4-dimethoxy-phenyl)-2-methylamino-ethanone; hydrochloridewith hydrogen;5%-palladium/activated carbon in water at 75; under 2585.81 Torr; for 16 - 40 h;
Stage #2: with sodium hydroxide in water;toluene; pH=12;Product distribution / selectivity;
Steps:
5
10 g of N-methyl-2-(3,4-dimethoxyphenyl)-2-oxy-ethylamine hydrochloride were dissolved in 100 ml of water. The mixture was heated up to 75° C. then 1 g of Pd/C 5% 50% wet was added to the mixture. The suspension so obtained was then hydrogenated under vigorous agitation (50 psi), at a temperature of 75° C. A sample withdrawn and analysed by HPLC after 16 hr shows 99.4% of the compound (5); <0.1% compound (4) and 0.4% of compound (6). After 24 hr the analytical profile shows (5)/(6)=99.2/0.6. After 40 hr the same ratio is 98.8/0.9.The catalyst was then recovered by filtration maintaining the temperature above 60° C. Then 50 ml of toluene were added and the mixture was treated with NaOH 30% (40 ml) till pH12 and clear solution was obtained. The clear aqueous phase, containing sodium aluminium hydroxides was discharged; the toluene solution was then concentrated under vacuum till residue.8.2 g of N-methyl-2-(3,4-dimethoxyphenyl)-2-hydroxy-ethylamine were recovered. Yield: 95.1%-purity 99.0% (compound 6 content=0.4%)
References:
US2009/171110,2009,A1 Location in patent:Page/Page column 6
![Benzenemethanol, 3,4-dimethoxy-α-[[methyl(phenylmethyl)amino]methyl]-](/CAS/20210305/GIF/345231-54-1.gif)
345231-54-1
0 suppliers
inquiry

5653-66-7
7 suppliers
$155.00/50mg
1835-02-5
106 suppliers
$7.00/1g

5653-66-7
7 suppliers
$155.00/50mg
![Ethanone, 1-(3,4-dimethoxyphenyl)-2-[methyl(phenylmethyl)amino]-](/CAS/20210305/GIF/33342-73-3.gif)
33342-73-3
0 suppliers
inquiry

5653-66-7
7 suppliers
$155.00/50mg
![Carbamic acid, [2-(3,4-dimethoxyphenyl)-2-hydroxyethyl]-, ethyl ester (9CI)](/CAS/20210305/GIF/54837-87-5.gif)
54837-87-5
0 suppliers
inquiry

5653-66-7
7 suppliers
$155.00/50mg