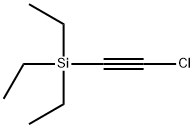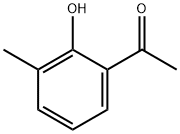
NSC 46633 synthesis
- Product Name:NSC 46633
- CAS Number:699-91-2
- Molecular formula:C9H10O2
- Molecular Weight:150.17
83-40-9
351 suppliers
$5.00/1g
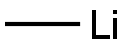
917-54-4
172 suppliers
$44.39/50ml

699-91-2
77 suppliers
$34.00/100mg
Yield: 90%
Reaction Conditions:
in diethyl ether at 0 - 20;Inert atmosphere;
Steps:
46.46.1 Step 46.1: 1-(2-hydroxy-3-methylphenyl)ethanone
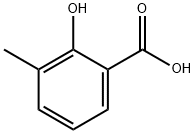
Step 46.1: 1-(2-hydroxy-3-methylphenyl)ethanone To a colorless solution of 3-methyl-salicylic acid (1 g, 6.57 mmol) in Et2O (50 mL) under argon and cooled down to 0° C. was added dropwise methyllithium 1.6M in Et2O (12.32 mL, 19.72 mmol) over 20 min. The resulting mixture was stirred at this temperature for 30 min then allowed to warm up and stir overnight at RT. The reaction mixture was slowly poured into a stirred mixture of 100 g of ice and HCl 4N (50 mL) and extracted twice with EtOAc. The combined organic layers were washed with 10% NaHCO3 solution and brine, dried over MgSO4, filtered and concentrated under reduced pressure to afford the title product (920 mg, 5.94 mmol, 90% yield) as a pale yellow oil. tR: 0.91 min (LC-MS 2); ESI-MS: 481/483 [M+H]+, ESI-MS: 479/501 [M-H]- (LC-MS 2); Rf=0.55 (hexane/EtOAc 3:1).
References:
NOVARTIS AG;BLANK, Jutta;BORDAS, Vincent;COTESTA, Simona;GUAGNANO, Vito;RUEEGER, Heinrich;VAUPEL, Andrea US2014/349990, 2014, A1 Location in patent:Paragraph 0739; 0740
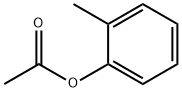
533-18-6
106 suppliers
$55.00/25g

699-91-2
77 suppliers
$34.00/100mg
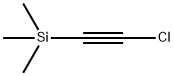
7652-06-4
3 suppliers
inquiry
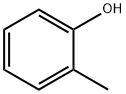
95-48-7
430 suppliers
$17.00/25g

699-91-2
77 suppliers
$34.00/100mg

99299-72-6
8 suppliers
inquiry

533-18-6
106 suppliers
$55.00/25g
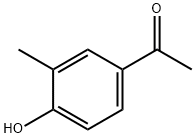
876-02-8
225 suppliers
$6.00/250mg

699-91-2
77 suppliers
$34.00/100mg