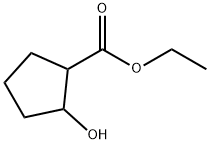
Nsc122560 synthesis
- Product Name:Nsc122560
- CAS Number:54972-10-0
- Molecular formula:C8H14O3
- Molecular Weight:158.2

611-10-9
400 suppliers
$10.00/5g

54972-10-0
24 suppliers
$220.00/100mg
Yield:54972-10-0 83.9%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 0; for 0.5 h;
Steps:
24 EXAMPLE 24 1-[2-(3,4-Dichlorophenyl)-3-(2-hydroxycyclopentyl)propionyl]-3-methylurea
[0230] A solution of 2-oxo-cyclopentanecarboxylic acid ethyl ester (10 g, 64.0 mmol) in ethanol (106.7 mL) cooled to 0° C. was treated with 98% sodium borohydride (686 mg, 17.78 mmol). The reaction was stirred at 0° C. for 30 min. At this time, the reaction mixture was poured into water (53 mL) and was extracted into diethyl ether (3×100 mL). The organics were dried over sodium sulfate, filtered, and concentrated in vacuo. Flash chromatography (Merck Silica gel 60, 230-400 mesh, 75/25 ethyl acetate/hexanes) afforded 2-hydroxy-cyclopentanecarboxylic acid ethyl ester (8.5 g, 83.9%) as a clear liquid. [0231] A solution of 2-hydroxy-cyclopentanecarboxylic acid ethyl ester (3.5 g, 22.12 mmol) in methylene chloride (147.5 mL) was treated with 3,4-dihydro-2H-pyran (3.03 mL, 33.1 mmol) and pyridinium p-toluenesulfonate (556 mg, 2.21 mmol). This solution was stirred at 25° C. for 5 h. The reaction was then washed with a half-saturated aqueous sodium chloride solution (2×75 mL), dried over sodium sulfate, filtered, and concentrated in vacuo. Flash chromatography (Merck Silica gel 60, 230-400 mesh, 90/10 hexanes/ethyl acetate) afforded 2-(tetrahydro-pyran-2-yloxy)-cyclopentanecarboxylic acid ethyl ester (4.7 g, 87.7%) as a clear liquid: EI-HRMS m/e calcd for C13H22O4 (M+) 242.1518 found 242.1521. [0232] A slurry of lithium aluminum hydride (883 mg, 23.27 mmol) in tetrahydrofuran (19.4 mL) cooled to 0° C. was treated with 2-(tetrahydro-pyran-2-yloxy)-cyclopentanecarboxylic acid ethyl ester (4.7 g, 19.59 mmol). The reaction was stirred at 25° C. for 18 h. At this time, the reaction was poured onto ice/water. This mixture was filtered through a pad of celite (methylene chloride as eluent). The organics were washed with a saturated aqueous sodium chloride solution (1×100 mL), dried over sodium sulfate, filtered, and concentrated in vacuo to give [2-(tetrahydro-pyran-2-yloxy)-cyclopentyl]-methanol (3.25 g, 83.6%) as a clear liquid: EI-HRMS m/e calcd for C11H20O3 (M+) 200.1412 found 200.1412. [0233] A solution of triphenylphosphine (1.70 g, 6.49 mmol) and imidazole (884 mg, 12.98 mmol) in methylene chloride (8.32 mL) cooled to 0° C. was treated with iodine (1.64 g, 6.49 mmol). After the iodine was completely dissolved, a solution of [2-(tetrahydro-pyran-2-yloxy)-cyclopentyl]-methanol (1.0 g, 4.99 mmol) was added to the reaction mixture. The reaction was stirred at 0° C. for 1 h and at 25° C. for 2 h. At this time, the reaction was poured into water (100 mL) and extracted with methylene chloride (1×30 mL). The organics were washed with a saturated aqueous sodium thiosulfate solution (1×50 mL), dried over sodium sulfate, filtered, and concentrated in vacuo at 25° C. Flash chromatography (Merck Silica gel 60, 230-400 mesh, 80/20 hexanes/ethyl acetate) afforded 2-(2-iodomethyl-cyclopentyloxy)-tetrahydropyran (1.17 g, 75.8%) as a clear liquid: EI-HRMS m/e calcd for C11H19IO2 (M+) 309.0352 found 309.0348. [0234] A solution of freshly prepared lithium diisopropylamide (10.4 mL of a 0.31M stock solution, 3.22 mmol) cooled to -78° C. was treated with 3,4-dichlorophenylacetic acid methyl ester (prepared as in Example 1, 642 mg 2.93 mmol) in tetrahydrofuran/1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (7.33 mL, 3:1). The resulting solution was stirred at -78° C. for 45 min. A solution of 2-(2-iodomethyl-cyclopentyloxy)-tetrahydropyran (1.0 g, 3.22 mmol) in 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (1 mL) was then added. The reaction mixture was stirred at -78° C. for 2 h. The reaction was then warmed to 25° C. and was stirred at 25° C. for 16 h. The reaction mixture was then quenched by the dropwise addition of a saturated aqueous ammonium chloride solution (10 mL). This mixture was poured into water (100 mL) and extracted with methylene chloride (3×50 mL). The organics were dried over sodium sulfate, filtered, and concentrated i
References:
US2003/225283,2003,A1 Location in patent:Page 28-29
141-28-6
351 suppliers
$10.00/100g

54972-10-0
24 suppliers
$220.00/100mg