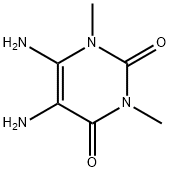
NSC14355 synthesis
- Product Name:NSC14355
- CAS Number:1784-70-9
- Molecular formula:C7H8N4O2S
- Molecular Weight:212.23
Yield:1784-70-9 80%
Reaction Conditions:
in N,N-dimethyl-formamide;Reflux;
Steps:
Synthesis of 8-thio-l,3-dimethyl-3,7-dihydro-lH-purine-2, 6-dione (4)
Compound 3 (1.00 g, 6.0 mmol) was stirred with 100 mL of DMF in an round bottom flask equipped with a stir bar. The solution was stirred at room temperature until completely dissolved and then CS2 (2.237 g, 30.0 mmol) was added to the mixture. The solution was refluxed for 6 hours. After confirming by TLC (5% MeOH/DCM), the solution was cooled in an ice bath and cold water was added, forming a white precipitate. The aqueous solution was filtered. The resulting solid was washed with cold water and ethyl ether to afford 8-thio-l,3-dimethyl-3,7-dihydro-lH-purine-2, 6-dione 4 in 80% yield: XH NMR (400 MHz, DMSO- 1/6) 6 13.39 (s, 1H), 12.98 (s, 1H), 3.35 (s, 3H), 3.16 (s, 3H); 13C NMR (100 MHz, DMSO- 1/6) 6 163.9, 151.6, 150.1, 139.4, 103.6, 31.2, 27.8.
References:
WO2022/159818,2022,A1 Location in patent:Paragraph 0188
6642-31-5
371 suppliers
$5.00/10g

1784-70-9
15 suppliers
inquiry

6632-68-4
117 suppliers
inquiry

1784-70-9
15 suppliers
inquiry
![2-cyano-N-methyl-N-[(methylamino)carbonyl]acetamide](/CAS/GIF/39615-79-7.gif)
39615-79-7
37 suppliers
inquiry

1784-70-9
15 suppliers
inquiry