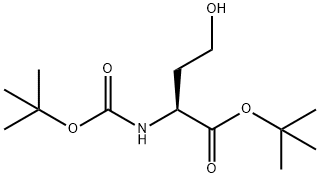
NW-09-15-1 synthesis
- Product Name:NW-09-15-1
- CAS Number:81323-58-2
- Molecular formula:C13H25NO5
- Molecular Weight:275.34
34582-32-6
242 suppliers
$6.00/1g

81323-58-2
51 suppliers
$100.00/25 mg
Yield: 95%
Reaction Conditions:
Stage #1:N-tert-butoxycarbonyl aspartic acid tert-butyl ester with 4-methyl-morpholine;isobutyl chloroformate in 1,2-dimethoxyethane at -15; for 0.166667 h;
Stage #2: with sodium tetrahydroborate;water in 1,2-dimethoxyethane at -15; for 0.0833333 h;
Steps:
1.b
1 b) tert-Butyl N-(tert-butoxycarbonyl)-L-homoserinate To a solution of Boc-L-Asp-OtBu (3.00 g, 10.4 mmol) in 1,2-dimethoxyethane (10 mL) was added at -15 °C 4-methylmorpholine (1.14 mL, 10.4 mmol) and isobutyl chloroformate (1.35 mL, 10.4 mmol). After stirring for 10 min at -15 °C the precipitate was filtered off and washed with cold 1,2-dimethoxyethane (20 mL). To the filtrate was added at -15 °C a solution of sodium borohydride (0.59 g, 15.6 mmol) in water (5 mL). After 5 min water (250 mL) was added. The reaction mixture was extracted with ethyl acetate (3 x 100 mL), the combined organic layers dried over sodium sulphate, and concentrated under reduced pressure to give the title compound. Yield: 2.70 g, 9.81 mmol, 95 %. MS (ESIpos): m/z = 276 [M+H]+ 1H-NMR (400MHz, CHLOROFORM-d): δ [ppm]= 1.46 (s, 9H), 1.48 (s, 9H), 1.51 - 1.58 (m, 1H), 2.09 - 2.20 (m, 1H), 3.45 (br. s., 1H), 3.55 - 3.76 (m, 2H), 4.31 - 4.40 (m, 1H), 5.35 (d, 1H).
References:
Bayer Schering Pharma Aktiengesellschaft EP2322500, 2011, A1 Location in patent:Page/Page column 26-27