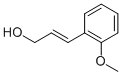
o-Methoxycinnamyl alcohol synthesis
- Product Name:o-Methoxycinnamyl alcohol
- CAS Number:1504-61-6
- Molecular formula:C10H12O2
- Molecular Weight:164.2011
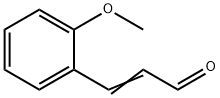
1504-74-1
176 suppliers
$7.00/100mg

1504-61-6
4 suppliers
inquiry
Yield:1504-61-6 99%
Reaction Conditions:
with Hf-based metal organic framework-808 in isopropanol at 120;Inert atmosphere;Meerwein-Ponndorf-Verley Reduction;
Steps:
2.3 The general procedure for the selective hydrogenation of ketones or aldehydes
General procedure: A mixture of 0.2 mmol of carbonyl compound and 20 mg of Hf-MOF was added to isopropanol (1 mL), and the mixture was stirred and reacted at 120 °C for 12 h under N2 atmosphere. After the reaction was completed, Hf-MOF was isolated from the solution by centrifugation and wash by EtOAc (1 mL × 3). Then the resulted solid was reused for another reaction cycle directly. The organic layer was collected and removed in vacuo to afford the crude product, which was analyzed by GC and GC-MS.
References:
Lin, Yamei;Bu, Qingxia;Xu, Jiaxian;Liu, Xiao;Zhang, Xueping;Lu, Guo-Ping;Zhou, Baojing [Molecular catalysis,2021,vol. 502,art. no. 111405]

33877-05-3
20 suppliers
$94.25/1g

1504-61-6
4 suppliers
inquiry
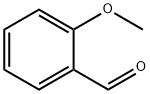
135-02-4
384 suppliers
$10.00/10g

1504-61-6
4 suppliers
inquiry
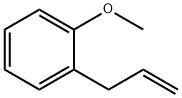
3698-28-0
26 suppliers
$121.00/5g

1504-61-6
4 suppliers
inquiry

15854-58-7
10 suppliers
inquiry

1504-61-6
4 suppliers
inquiry