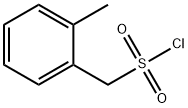
O-TOLYL-METHANESULFONYL CHLORIDE synthesis
- Product Name:O-TOLYL-METHANESULFONYL CHLORIDE
- CAS Number:92614-55-6
- Molecular formula:C8H9ClO2S
- Molecular Weight:204.67
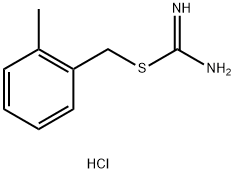
73338-92-8
9 suppliers
inquiry

92614-55-6
52 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with hydrogenchloride;N-chloro-succinimide in water;acetonitrile at 0 - 20; for 0.5 h;
Steps:
4.1.3. General procedure for preparation of sulfonyl azides 24a-k
General procedure: Various alkyl halides 23a-k (2.5 mmol) and thiourea (0.19 g, 2.5 mmol) were refluxed together in EtOH (2.5 mL) for 1 h. Afterr emoval of EtOH at reduced pressure, the obtained solid was slowly added to a mixture of NCS (1.33 g, 10 mmol), 2 M HCl (0.68 mL) and MeCN (4 mL) over 15 min. Upon completion of the reaction (TLC monitoring), the solvent was removed under vacuum. Then H2O(5 mL)was added to the residue and stirred for 15 min, the resulting solid was filtered and dried under an infrared lamp to afford the corresponding sulfonyl chlorides 24a-k. Without further purification, 24a-k was added in portions to a solution of NaN3 (195 mg ,3 mmol) in acetone/H2O (10 mL, acetone/H2O = 1:1) at 0 °C. After being stirred at room temperature for 4 h, the reaction mixtur ewas concentrated in vacuo and H2O (10 mL) was added to the residue. The aqueous solution was extracted with CHCl3 (3 x 10 mL). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure to afford sulfonyl azides 25a-k.
References:
Fang, Sen-Biao;Li, Hui-Jing;Nan, Xiang;Wu, Rui;Wu, Yan-Chao;Zhang, Jing;Zhang, Zhi-Zhou [European Journal of Medicinal Chemistry,2020,vol. 200]

552-45-4
331 suppliers
$21.00/25g

92614-55-6
52 suppliers
$45.00/50mg