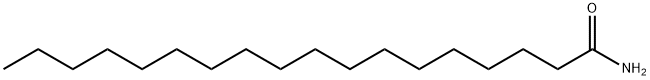
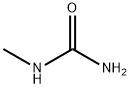
OctadecanaMide, N-Methyl- synthesis
- Product Name:OctadecanaMide, N-Methyl-
- CAS Number:20198-92-9
- Molecular formula:C19H39NO
- Molecular Weight:297.52
Yield:20198-92-9 65%
Reaction Conditions:
with n-butyllithium;cyclopentyl methyl ether in hexane at 115; for 24 h;Inert atmosphere;Reagent/catalyst;
Steps:
Typical procedure B (To reverse the adding order of the base and phosphate derivative compared to the Typical procedure A.)
General procedure: To a solution of the amide derivative (1: 0.20 mmol) in CPME (1.0 mL) wassequentially added trialkyl phosphate (0.60 mmol, 3.0 equiv.) and n-BuLi (0.14 mL,0.37 mmol, 2.65 mol/L in n-hexane, 1.8 equiv.). After stirring at 115 °C under argon for24 h, the mixture was quenched with brine (2 mL) and extracted with EtOAc (50 mL ×3). The combined organic layers were dried over Na2SO4 and concentrated in vacuo.The residue was purified by silica-gel column chromatography to give the N-mono alkylamide product.
References:
Asai, Shota;Ban, Kazuho;Monguchi, Yasunari;Sajiki, Hironao;Sawama, Yoshinari [Synlett,2018,vol. 29,# 3,art. no. ST-2017-U0636-L,p. 322 - 325] Location in patent:supporting information