
Octadecanoic acid, 18-bromo- synthesis
- Product Name:Octadecanoic acid, 18-bromo-
- CAS Number:2536-38-1
- Molecular formula:C18H35BrO2
- Molecular Weight:363.37

59101-17-6
7 suppliers
inquiry

2536-38-1
27 suppliers
inquiry
Yield:2536-38-1 98%
Reaction Conditions:
with Jones reagent in water;acetone; for 1.25 h;Cooling;
Steps:
2.B Compound 20.
Compound 20. (0421) A round bottom flask was charged with compound 19 (2.01 g, 5.73 mmol) and the solid dissolved in reagent grade acetone (150 mL). Simultaneously, Jones reagent was prepared by dissolving CrO3 (2.25 g, 22 mmol) in H2SO4 (4 mL) and then slowly adding 10 mL of cold water and letting the solution stir for 10 min. The cold Jones reagent was then added to the round bottom flask slowly over 5 min., after which the solution stirred for 1 hr. The resulting orange solution turned green within several minutes. The mixture was then quenched with water (150 mL) extracted twice in ether (3×150 mL). The ether layers were then dried with magnesium sulfate, and concentrated to yield compound 20 (2.08 g, 98% yield) as a white powder. 1H NMR (CDCl3, 200 MHz) δ 1.27 (br s, 26H), 1.58-1.71 (m, 2H), 1.77-1.97 (m, 2H), 2.36 (t, 2H, J=7.4 Hz), 3.42 (t, 2H, J=7 Hz).
References:
US9163091,2015,B2 Location in patent:Page/Page column 61; 63
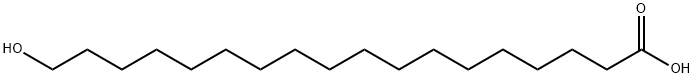
3155-42-8
38 suppliers
inquiry

2536-38-1
27 suppliers
inquiry

55362-80-6
277 suppliers
$10.00/1g

2536-38-1
27 suppliers
inquiry
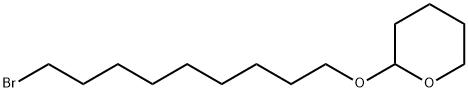
55695-90-4
9 suppliers
$500.26/5MG

2536-38-1
27 suppliers
inquiry
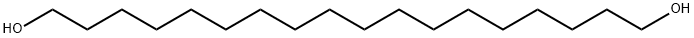
3155-43-9
123 suppliers
$24.00/1g

2536-38-1
27 suppliers
inquiry