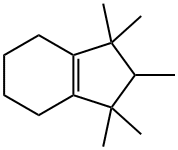
octahydro-1,1,2,3,3-pentamethyl-1H-indene synthesis
- Product Name:octahydro-1,1,2,3,3-pentamethyl-1H-indene
- CAS Number:33704-60-8
- Molecular formula:C14H26
- Molecular Weight:194.36
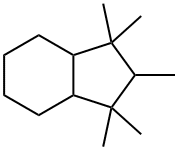
1203-17-4
2 suppliers
inquiry

33704-60-8
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen in decalin at 200; under 34375.6 Torr; for 3 h;Autoclave;Reagent/catalyst;Time;Temperature;
Steps:
1 Example 1: Use of Carbon as a Support for Pd Catalysts Compared to Other Supports, Such as Silica, Alumina, Silica-Alumina and Titania
Catalyst 1 (0016) 5 wt % Pd/silica was synthesized by dissolving the precursor, palladium(II) nitrate hydrate, Pd(NO3)2.xH2O (Sigma Aldrich 205761-2G), in deionized water to make a single solution. The solution was then deposited onto a support using the incipient wetness impregnation method. For Catalyst 1, the support was silica (Saint-Gobain NorPro SS 61138). The solution was added dropwise to the support with continuous mixing and stirring. After the metal deposition, the sample was dried in an oven in static air at 120° C. overnight (12 hours) and used for testing without any additional pretreatment (without calcination or reduction). Catalyst 2 (0017) 5 wt % Pd/titania was synthesized using the same procedure as Catalyst 1, with the exception that the support was titania (Saint-Gobain NorPro ST 61120). Catalyst 3 (0018) 5 wt % Pd/fumed silica was synthesized using the same procedure as Catalyst 1, with the exception that the support was fumed silica (Cabot CAB-O-SIL HS-5). Catalyst 4 (0019) 5 wt % Pd/alumina was synthesized using the same procedure as Catalyst 1, with the exception that the support was alumina (Saint-Gobain NorPro SA 6175). Catalyst 5 (0020) 5 wt % Pd/silica-alumina was synthesized using the same procedure as Catalyst 1, with the exception that the support was silica-alumina (Saint-Gobain NorPro SS 61155). (0021) Catalyst 6 (0022) 5 wt % Pd/carbon was synthesized using the same procedure as Catalyst 1, with the exception that the support was acid-washed activated carbon (Cabot Norit SX 2). (0023) Catalysts 1-6 were tested by hydrogenating 1,1,2,3,3-pentamethyl indane (PMI) to 1,1,2,3,3-pentamethyl-tetrahydro indane (THPMI) and further to 1,1,2,3,3-pentamethyl-hexahydro indane (HHPMI) using the following protocol: (0024) 1. A 300 mL Parr reactor was loaded with 40.0 g of PMI and 120.0 g of decahydronaphthalene (decalin) (with a PMI to decalin mass ratio of 1 to 3). 1 wt % of a solid catalyst (0.40 g) was added to the liquid. (0025) 2. The reactor was flushed with N2 twice and checked for leaks. (0026) 3. The reactor was filled with H2 at 100 psi and checked for leaks. (0027) 4. The reactor H2 pressure was increased to 400 psig, and mixing started with an agitation speed of 700 rpm. (0028) 5. Temperature was raised to the desired testing temperature of 200° C. and held constant for the duration of the test. (0029) 6. After reaching the desired testing temperature, the reactor H2 pressure was increased to 650 psig. This point of the pressure increase to 650 psig was taken as zero time on stream. (0030) 7. Liquid samples from the reactor were collected every 30 min and analyzed using a gas chromatograph (GC) equipped with a flame ionization detector and a Carbowax HP-5 column.
References:
US2020/1274,2020,A1 Location in patent:Paragraph 0016-0030; 0035; 0039; 0043; 0047; 0051; 0058