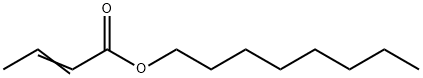
OCTYL-CROTONATE synthesis
- Product Name:OCTYL-CROTONATE
- CAS Number:22874-79-9
- Molecular formula:C12H22O2
- Molecular Weight:198.3

111-87-5
730 suppliers
$6.00/100g

107-93-7
308 suppliers
$11.19/100G

22874-79-9
12 suppliers
inquiry
Yield:22874-79-9 273 g
Reaction Conditions:
with sodium tetrahydroborate in toluene at 120 - 130;Dean-Stark;
Steps:
XI Preparation of But-2-enoic acid octyl ester (Structure XI)
Example XI
Preparation of But-2-enoic acid octyl ester (Structure XI)
Octan-1-ol (221 g), crotonic acid (194 g), PTSA (6.4 g), and toluene (300 mL) were charged into a 2-L reaction flask fitted with a mechanical stirrer, a thermocouple, a Dean-Stark trap, and a condenser.
The reaction mixture was heated to reflux at about 120-130° C. Water was removed azeotropically.
The reaction was aged at reflux for about 7-8 hours until no more water evolved.
The reaction mixture was cooled to room temperature and quenched with water (400 mL).
The organic layer was separated and subsequently washed with sodium carbonate (5%, 300 mL), and further fractional distilled to provide but-2-enoic acid octyl ester (273 g) with a boiling point of 123° C. at 11 mmHg.
1H NMR (CDCl3, 500 MHz): 6.96 ppm (d, 1H, J=15.52 Hz, of q, J=6.92 Hz), 5.84 ppm (d, 1H, J=15.52 Hz, of q, J=1.66 Hz), 4.11 ppm (t, 2H, J=6.74 Hz), 1.87 ppm (d, 3H, J=6.92 Hz, of d, J=1.66 Hz), 1.60-1.70 ppm (m, 2H), 1.23-1.42 ppm (m, 10H), 0.88 ppm (t, 3H, J=6.84 Hz)
References:
US8772533,2014,B1 Location in patent:Page/Page column 15; 16
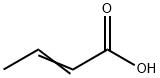
3724-65-0
186 suppliers
$1146.05/25G

111-87-5
730 suppliers
$6.00/100g

22874-79-9
12 suppliers
inquiry

107-93-7
308 suppliers
$11.19/100G

22874-79-9
12 suppliers
inquiry