
Oleoyl Serotonin synthesis
- Product Name:Oleoyl Serotonin
- CAS Number:1002100-44-8
- Molecular formula:C28H44N2O2
- Molecular Weight:440.66
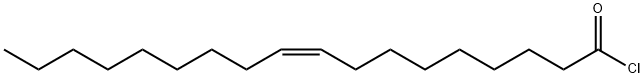
112-77-6
256 suppliers
$21.00/1g

50-67-9
169 suppliers
$106.00/25mg

1002100-44-8
19 suppliers
$43.00/5mg
Yield:1002100-44-8 82%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 20; for 5 h;Cooling with ice;
Steps:
General Procedure for Preparation of N-Acylhistamines (N-Acyl-HAs)
General procedure: A solution of fatty acid acyl chloride (1.0 mmol), purchased from Tokyo Chemical Industory (Tokyo, Japan), in N,N-dimethylformamide (DMF) (2 mL) was added dropwise to a suspension of histamine dihydrochlolide (2.0 mmol) and Et3N (8 mmol) in DMF (5 mL) cooled in an ice bath. In some cases (i.e., for the C18:1, C18:2 and C20:4 analogues), the acyl chlorides were prepared by reacting the free fatty acids with oxalyl chloride (5 eq, CH2Cl2, r.t., 3 h). The reaction mixture was stirred for 5 h at room temperature. Ice water was added to the mixture and the reaction mix was extracted with CHCl3. The organic layer was dried over Na2SO4 and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3 : MeOH : aq. NH3=20 : 1 : 0.5) to give the corresponding N-acylhistamine.
References:
Takao, Koichi;Noguchi, Kaori;Hashimoto, Yosuke;Shirahata, Akira;Sugita, Yoshiaki [Chemical and Pharmaceutical Bulletin,2015,vol. 63,# 4,p. 278 - 285]
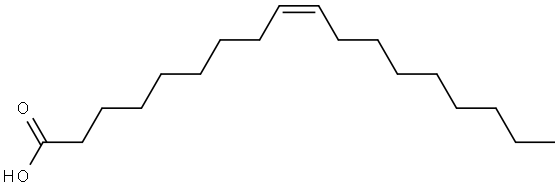
112-80-1
993 suppliers
$5.00/10g
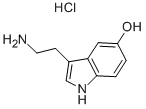
153-98-0
354 suppliers
$32.00/50mg

1002100-44-8
19 suppliers
$43.00/5mg

112-80-1
993 suppliers
$5.00/10g

1002100-44-8
19 suppliers
$43.00/5mg