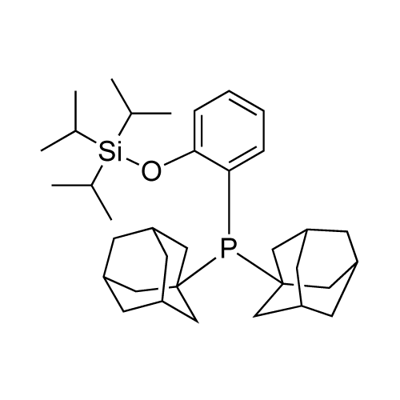
OTIPS-DalPhos synthesis
- Product Name:OTIPS-DalPhos
- CAS Number:1384966-55-5
- Molecular formula:C35H55OPSi
- Molecular Weight:550.87

131211-27-3
142 suppliers
$40.00/1g
378787-34-9
5 suppliers
$16.10/1g

1384966-55-5
15 suppliers
$149.00/250mg
Yield:1384966-55-5 91%
Reaction Conditions:
with 1,1'-bis(diisopropylphosphino)ferrocene;palladium diacetate;sodium t-butanolate in toluene at 110; for 12 h;Inert atmosphere;Sealed tube;Concentration;
Steps:
7 General P-C cross-coupling procedure:
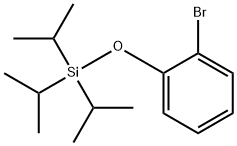
To an oven dried screw-capped vial was added a stir bar, (2-bromophenoxy)- silane (2.1 mmol), Pd(OAc)2 (3 mol%), l,l'bis(diisopropylphosphino)ferrocene (3.5 mol%), NaOt-Bu (3 equivalents) and 5 mL of toluene. The resulting suspension was stirred until apparently homogeneous and then di(l-adamantyl)phosphine (645 mg, 2.1 mmol) was added. [00184] The vial was sealed under dinitrogen with a cap containing a PTFE septum, removed from the glove box, placed in a temperature-controlled aluminum heating block set at 110°C and vigorous magnetic stirring was initiated. After 12 h, 31P NMR analysis of the reaction mixture confirmed the consumption of di(l-adamantyl)phosphine and the quantitative formation of one new phosphorus-containing product. The vial containing the reaction mixture was then cooled and opened to air; the reaction mixture was then filtered through a plug of silica, which in turn was washed with methylene chloride. Removal of the solvent from the combined eluent afforded the target ligand, which were further purified by recrystallization from cold hexanes as a range of off-white powders.
References:
WO2013/159229,2013,A1 Location in patent:Paragraph 00182-00184