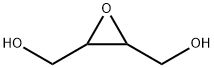
oxirane-2,3-dimethanol synthesis
- Product Name:oxirane-2,3-dimethanol
- CAS Number:4440-87-3
- Molecular formula:C4H8O3
- Molecular Weight:104.1045
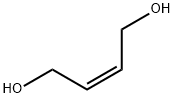
6117-80-2
183 suppliers
$10.00/5g

4440-87-3
9 suppliers
inquiry
Yield: 85%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in acetonitrile at 0 - 20; for 24 h;
Steps:
4 Part A: Synthesis of Monomer Precursor
Preparation of a cold solution of 7 (Z)-but-2-ene-1,4-diol (50 g, 567.47 mmol) in 8 CH3CN (650 mL) was added 9 m-chloroperbenzoic acid (144 g, 584.49 mmol). The mixture was stirred at from 0° C. to rt for 24 h. The 10 benzoic acid formed was removed by filtration and the filtrate was washed with diethyl ether to remove the unreacted m-chloroperbenzoic acid and the remaining chlorobenzoic acid. The product was isolated from the solution by diethyl ether to provide 51.5 g (85%). 12 Oxirane 2,3 13 diyldimethanol (5 g, 48.02 mmol), 14 methacrylic acid (8.68 g, 100.84 mmol, 15 tri-ethyl amine (0.7 ml, 0.4.80 mmol), 16 BHT (catalyst amounts) were added to 17 CH3CN in a RBF fitted with a condenser. The reaction mixture was stirred for 48 h at 50° C. After the reaction mixture evaporated under reduced pressure and washed with diethyl ether to remove the residual methacrylic acid. The product 18 1,3,4-trihydroxybutan-2-yl methacrylate was obtained.
References:
Rohm and Haas Electronic Materials Korea Ltd.;Ryu, Eui Hyun;Jang, Min Kyung;Kim, Jung Woo;Choi, Kwang-Mo;Jeon, Hyun;Lee, Woo-Hyung;Kim, Myung-Yeol US2018/59545, 2018, A1 Location in patent:Paragraph 0101

821-11-4
110 suppliers
$11.00/250mg

4440-87-3
9 suppliers
inquiry