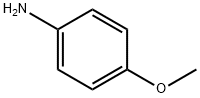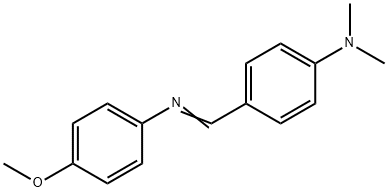
P-DIMETHYLAMINOBENZYLIDENE P-ANISIDINE synthesis
- Product Name:P-DIMETHYLAMINOBENZYLIDENE P-ANISIDINE
- CAS Number:1749-04-8
- Molecular formula:C16H18N2O
- Molecular Weight:254.33
Yield:1749-04-8 93%
Reaction Conditions:
with sulfuric acid in neat (no solvent);Microwave irradiation;Sealed tube;Green chemistry;
Steps:
synthesis of imines
General procedure: An appropriate equi-molar quantities of aryl amines (2 mmol), substituted benzaldehydes (2 mmol) and fly-ash: H2SO4 (0.5 g) have been taken in ACE tube and tightly capped. The mixture has been subjected to microwave irradiation for 6-12 min in a microwave oven (Scheme 1) (LG Grill, Intellowave, Microwave Oven, 160-800 W) and then cooled to room temperature. After separating the organic layer with dichloromethane the solid product has been obtained on evaporation. The solid, on recrystallization from benzene-hexane mixture afforded glittering product. The insoluble catalyst has been recycled by washing with ethyl acetate (8 mL) followed by drying in an oven at 100 °C for 1 h and reused for further reactions.
References:
Suresh;Kamalakkannan;Ranganathan;Arulkumaran;Sundararajan;Sakthinathan;Vijayakumar;Sathiyamoorthi;Mala;Vanangamudi;Thirumurthy;Mayavel;Thirunarayanan [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2013,vol. 101,p. 239 - 248]
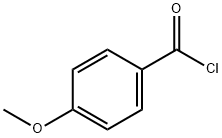
100-07-2
388 suppliers
$35.00/25g

1749-04-8
19 suppliers
$100.00/1g
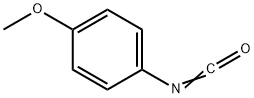
5416-93-3
139 suppliers
$14.14/250mgs:
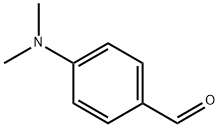
100-10-7
633 suppliers
$5.00/1g

1749-04-8
19 suppliers
$100.00/1g