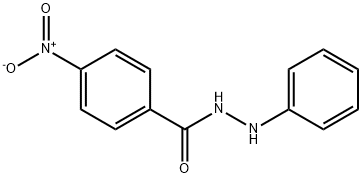
p-Nitrobenzoic acid 2-phenylhydrazide synthesis
- Product Name:p-Nitrobenzoic acid 2-phenylhydrazide
- CAS Number:39718-99-5
- Molecular formula:C13H11N3O3
- Molecular Weight:257.24
Yield:39718-99-5 94%
Reaction Conditions:
with pyridine in tetrahydrofuran at 5 - 20; for 2 h;
Steps:
28 Example 28. 4-nitro-N'-phenylbenzohydrazide (28)
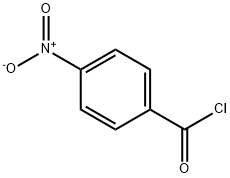
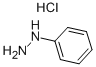
Following the general procedure Route C, 4-nitro-N'-phenylbenzohydrazide was obtained from the coupling reaction of 4-nitrobenzoyl chloride (2.78 g, 15 mmol) and phenylhydrazine (1 .78 g, 16.5 mmol), in presence of pyridine (2.65 mL, 33 mmol). Phenylhydrazine is solubilized in pyridine in a 50 mL flask and cooled at 5 ° C with an ice bath. In parallel, 4-nitrobenzoyl chloride is dissolved in 5 mL of THF. The acyl chloride solution is then added dropwise at 5 °C to the phenylhydrazine solution. After addition, the temperature is allowed to warm to room temperature and the medium is stirred during 2 hours. Pyridinium chloride gradually appeared in the medium. After completion of the reaction, water (30 mL) is added, pyridinium chloride dissolved and 4-nitro-N'-phenylbenzohydrazide precipitated. The precipitate is filtered, washed by diethylether (3x10 mL) and dried. Compound 28 is obtained as an orange solid (3.62 g, 94% yield), (0437) m.p. 142-143 ° C (0438) 1H NMR (400 MHz, DMSO-d6): d 10.69 (s, 1 H, N H), 8.35 (d, 2 H, J = 9.2 Hz, CHAr), 8.16 (d, 2 H, J = 8.4 Hz, CHAr), 8.03 (s, H, NH), 7.17 (t, J = 8.4 Hz, 2H, CHAr), 6.82 (d, J = 8.4 Hz, 2H, CHAr), 6.74 (t, J = 7.6 Hz, 1 H, CHAr). (0439) 13C{1H}NMR (100 MHz, DMSO-cU): 165.2 (C=0), 149.7 (C,v), 149.5 (C,v), 139.1 (C,v), 129.3 (2CHAr), 129.2 (2CHAr), 124.1 (2CHAr), 119.3 (CH), 1 12.8 (2CHAr). (0440) IR v (cm'1): 3285, 1635, 1597, 1545, 1342, 871 , 726, 692.
References:
WO2020/169682,2020,A1 Location in patent:Page/Page column 41-42
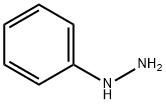
100-63-0
364 suppliers
$10.00/1g
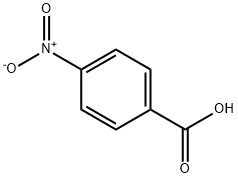
62-23-7
691 suppliers
$14.00/25g

39718-99-5
3 suppliers
inquiry

55685-55-7
0 suppliers
inquiry