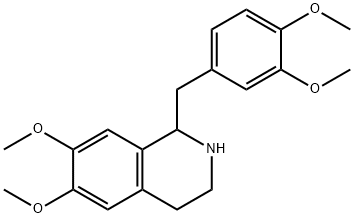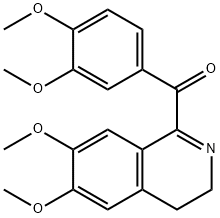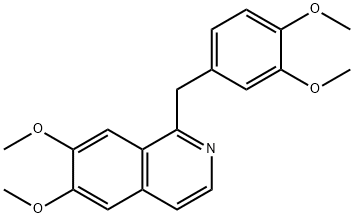
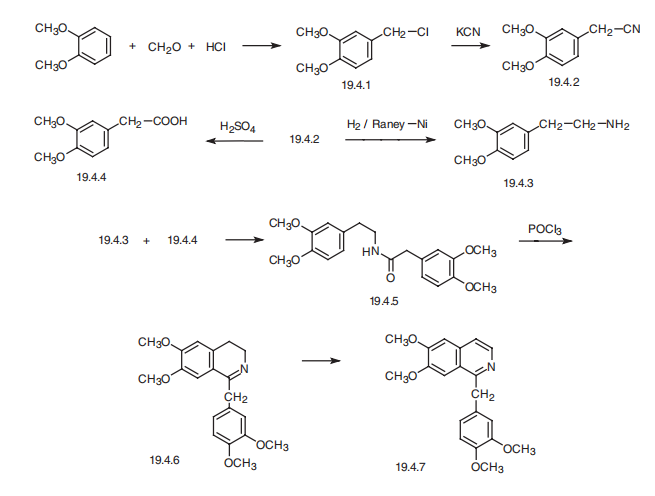
PAPAVERINE HYDROCHLORIDE synthesis
- Product Name:PAPAVERINE HYDROCHLORIDE
- CAS Number:58-74-2
- Molecular formula:C20H21NO4
- Molecular Weight:339.39
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

58-74-2
4 suppliers
$75.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
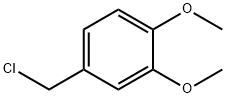
7306-46-9
171 suppliers
$22.00/100mg

58-74-2
4 suppliers
$75.00/10mg

482-76-8
19 suppliers
inquiry

58-74-2
4 suppliers
$75.00/10mg
![1-[(3,4-Dimethoxyphenyl)methyl]-3,4-dihydro-6,7-dimethoxyisoquinoline](/CAS/GIF/6957-27-3.gif)