
Perfluoro-1-iodohexane synthesis
- Product Name:Perfluoro-1-iodohexane
- CAS Number:355-43-1
- Molecular formula:C6F13I
- Molecular Weight:445.95
Yield:-
Reaction Conditions:
with bis(pentafluoropropionyl)peroxide at 28 - 70; for 0.333333 h;Concentration;Temperature;Time;
Steps:
1
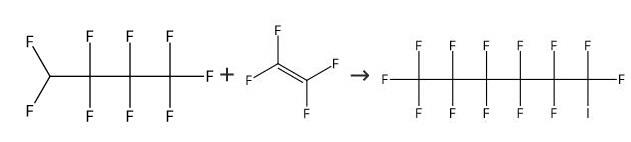
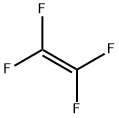
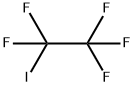
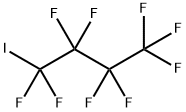
Into an autoclave 1 (made of stainless steel, capacity: 1 L) having a stirrer, C4F9I as the compound (1) was supplied at a flow rate of 25 mL/min, CF2=CF2 (tetrafluoroethylene) was supplied at a flow rate of 0.10 g/min, and (C2F5COO)2 (10 hour half-life temperature: 28°C) was supplied at a flow rate of 0.0065 mmol/min, and they were mixed by the stirrer to prepare a mixed solution. The temperature in the autoclave was set to be 70°C, and the retention time of the mixed solution in the autoclave was set to be 20 minutes. The obtained reaction mixture (1) contained C6F13I as the compound (2). The amounts of tetrafluoroethylene and (C2F5COO)2 were 0.65 mol% and 0.00425 mol%, respectively, to C4F9I.[0071] From the bottom of the autoclave 1, the reaction mixture (1) was withdrawn and supplied into an autoclave 2 (made of stainless steel, capacity: 1 L) having a stirrer, at a flow rate of 25 mL/min, CF2=CF2 (tetrafluoroethylene) was supplied at a flow rate of 0.10 g/min, and (C2F5COO)2 (10 hour half-life temperature: 28°C) was supplied at a flow rate of 0.0065 mmol/min, and they were mixed by the stirrer to prepare a mixed solution. The temperature in the autoclave was set to be 70°C, and the retention time of the mixed solution in the autoclave was set to be 20 minutes to obtain a reaction mixture (2) containing C6F13I as the compound (2). The amounts of tetrafluoroethylene and (C2F5COO)2 supplied, were 0.65 mol% and 0.00425 mol%, respectively, to the total of telomers in the mixed solution.[0072] From the bottom of the autoclave 2, the reaction mixture (2) was withdrawn, and an analysis of the composition was carried out to obtain the C4F9I conversion and C8+/C6 (%). The results are shown in Table 1.
References:
EP2578560,2013,A1 Location in patent:Paragraph 0070; 0071; 0072
116-14-3
45 suppliers
inquiry
354-64-3
162 suppliers
$45.00/5G

507-63-1
249 suppliers
$5.00/1g
423-39-2
235 suppliers
$6.00/5g

355-43-1
235 suppliers
$10.00/10g

423-62-1
145 suppliers
$10.00/1g

116-14-3
45 suppliers
inquiry

354-64-3
162 suppliers
$45.00/5G

507-63-1
249 suppliers
$5.00/1g

307-60-8
47 suppliers
$32.00/1g

355-43-1
235 suppliers
$10.00/10g

423-62-1
145 suppliers
$10.00/1g

116-14-3
45 suppliers
inquiry

354-64-3
162 suppliers
$45.00/5G

507-63-1
249 suppliers
$5.00/1g

423-39-2
235 suppliers
$6.00/5g

355-43-1
235 suppliers
$10.00/10g
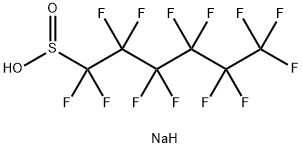
102061-83-6
1 suppliers
inquiry

355-43-1
235 suppliers
$10.00/10g