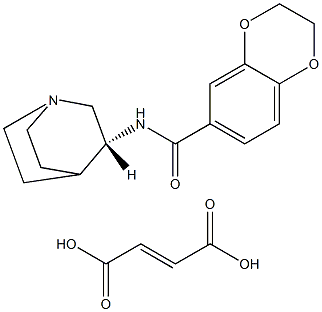
PHA 568487 synthesis
- Product Name:PHA 568487
- CAS Number:527680-57-5
- Molecular formula:C20H24N2O7
- Molecular Weight:404.4138
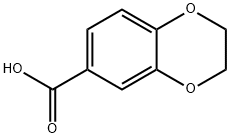
4442-54-0
205 suppliers
$7.00/500mg
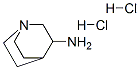
6530-09-2
153 suppliers
$6.00/250mg

527680-57-5
10 suppliers
inquiry
Yield:527680-57-5 634 mg (71%)
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in acetonitrile;
Steps:
1 N-((3R)-1-azabicyclo[2.2.2]oct-3-yl)-2,3-dihydro-1,4-benzodioxine-6-carboxamide fumarate
EXAMPLE 1 N-((3R)-1-azabicyclo[2.2.2]oct-3-yl)-2,3-dihydro-1,4-benzodioxine-6-carboxamide fumarate To a stirred solution of 0.59 g (3.3 mmol) of 1,4-benzodioxane-6-carboxylic acid in CH3CN (30 mL) in a -10° C. methanol-ice bath is added sequentially DIEA (1.65 mL, 9.5 mmol), 3(R)-aminoquinuclidine dihydrochloride (0.62 g, 3.11 mmol) and HATU (1.18 g, 3.11 mmol). The mixture is stirred at -10° C. for 1 h, followed by warming to rt and stirring overnight. The mixture is concentrated in vacuo to a yellow residue. The crude product is purified by flash chromatography on SiO2. Elution with CHCl3-MeOH-NH4OH (90:9:1) gave 634 mg (71%) of a light yellow solid. MS(ESI) m/e 289 [M+H].
References:
US2003/130305,2003,A1