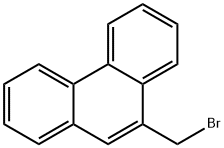
Phenanthrene, 9-(bromomethyl)- synthesis
- Product Name:Phenanthrene, 9-(bromomethyl)-
- CAS Number:24471-57-6
- Molecular formula:C15H11Br
- Molecular Weight:271.15

4707-72-6
34 suppliers
$155.00/10mg

24471-57-6
0 suppliers
inquiry
Yield:24471-57-6 79%
Reaction Conditions:
with phosphorus tribromide in dichloromethane at 0; for 2 h;
Steps:
4.2.21. 9-(Bromomethyl)phenanthrene (26)
Phosphorus tribromide (4.6 mL, 24.0 mmol)was slowly added toa solution of 25 (10.0 g, 48.0 mmol) in DCM (100 mL) at 0 °C. Thereaction mixture was stirred at 0 °C for 2 h. The reaction mixturewas sequentially washed with 5% NaHCO3 aqueous solution(100 mL), 10% sodium thiosulfate solution (100 mL) and water(100 mL). The separated organic layer was dried over sodium sulfateand concentrated under reduced pressure to afford 26. Yield 10.28 g (79%), mp 118-120 °C; 1H NMR (DMSO-d6)δ 5.27 (s, 2H,CH2), 7.66-7.79 (m, 4H, ArH), 7.98-7.99 (m, 1H, ArH), 8.08 (s, 1H,ArH), 8.25-8.27 (m, 1H, ArH), 8.82-8.84 (m, 1H, ArH), 8.89-8.91(m, 1H, ArH); 13C NMR (DMSO-d6) δ 33.57, 122.90, 123.47, 124.81,126.95, 127.08, 127.23, 127.71, 128.68, 128.86, 129.08, 130.18, 130.35,130.72, 131.99; HRMS [ESI+] calcd for C15H11Br, 271.0122 [M+H]+,found 271.0182.
References:
Jain, Vicky;Lai, Kuo-Chu;Lee, Te-Chang;Lin, Yi-Wen;Patel, Anilkumar S.;Rao, Vaikar Navakanth;Shah, Anamik;Su, Tsann-Long [European Journal of Medicinal Chemistry,2020,vol. 202]
883-20-5
31 suppliers
$620.00/1g

24471-57-6
0 suppliers
inquiry

82491-33-6
0 suppliers
inquiry

24471-57-6
0 suppliers
inquiry
4707-71-5
124 suppliers
$46.50/1g

24471-57-6
0 suppliers
inquiry
![1H-Cyclopropa[l]phenanthrene, 1a,9b-dihydro-](/CAS/20200611/GIF/949-41-7.gif)