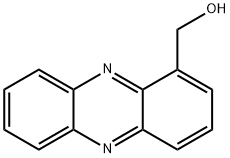
phenazin-1-ylmethanol synthesis
- Product Name:phenazin-1-ylmethanol
- CAS Number:1082-79-7
- Molecular formula:C13H10N2O
- Molecular Weight:210.23
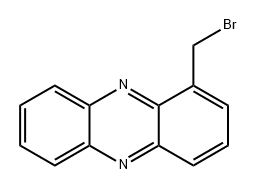
1019-54-1
1 suppliers
inquiry

1082-79-7
2 suppliers
inquiry
Yield:1082-79-7 98.4%
Reaction Conditions:
with sodium carbonate in water;N,N-dimethyl-formamide at 80; for 2 h;Solvent;Reagent/catalyst;Temperature;Time;Concentration;
Steps:
Synthesis of 1-phenazinemethanol (II)
Compound III (10 g, 36.6 mmol) was dissolved in the mixture of DMF (80mL) and 0.5 % Na2CO3 aqueous solution (88 mL) in a three necked flask equipped with an efficient reflux condenser. The mixture was heated at 80 C for 2 h under stirring. Then, the mixture was cooled and poured into water (100 mL) and extracted with EtOAc (50 mL ~ 3). The obtained EtOAc extract was washed with 0.2 N HCl (30 mL ~ 2) and finally washed with saturated NaCl solution. After drying with anhydrous Na2SO4, the organic layer was concentrated in vacuum to get the crude product. The residue was purified by recrystallization to obtain II (7.6 g, 98.4 %) as yellow solid. m.p. 132-134 C; 1H NMR (CDCl3, 400 MHz) : 4.50 (t, J = 6.4, 1H), 5.33 (d,J = 6.3, 2H), 7.72-7.78 (m, 2H), 7.84 (t, J = 4.1, 2H), 8.14-8.24 (m, 3H); 13C NMR (CDCl3, 100 MHz) : 143.7, 142.4,141.9, 138.8, 138.7, 130.7, 130.6, 130.3, 129.6, 129.5, 129.2,128.2, 63.9; ESI-MS m/z: 211.1 (100 %).
References:
Zhan;Zhu;Huang;Liu;Zhu [Asian Journal of Chemistry,2015,vol. 27,# 9,p. 3355 - 3360]

3225-19-2
6 suppliers
inquiry

1082-79-7
2 suppliers
inquiry
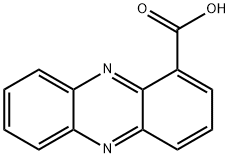
2538-68-3
113 suppliers
$116.00/100mg

1082-79-7
2 suppliers
inquiry
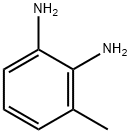
2687-25-4
280 suppliers
$16.00/1g

1082-79-7
2 suppliers
inquiry
120-80-9
494 suppliers
$13.00/25g

1082-79-7
2 suppliers
inquiry