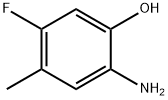
Phenol, 2-amino-5-fluoro-4-methyl- (9CI) synthesis
- Product Name:Phenol, 2-amino-5-fluoro-4-methyl- (9CI)
- CAS Number:83341-37-1
- Molecular formula:C7H8FNO
- Molecular Weight:141.14
83341-28-0
52 suppliers
$55.00/50mg

83341-37-1
12 suppliers
inquiry
Yield:83341-37-1 88%
Reaction Conditions:
with hydrogen at 25; under 75007.5 Torr;
Steps:
22
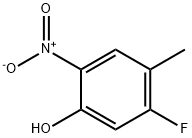
5-Fluoro-4-methyl-2-nitrophenol (D21 , 162 mg, 0.946 mmol) was reduced using H-Cube hydrogenator (full H2 mode, 25 0C, 1 ml_/min). The reaction was concentrated by rotary evaporation to give 2-amino-5-fluoro-4-methylphenol (D22, 117 mg, 88 % yield) as a pale yellow solid.1H NMR δ (CDCI3, 400 MHz) 2.13 (3 H, s), 6.51 (1 H, d, J 10), 6.61 (1 H, d, J 7.6).
References:
WO2009/37294,2009,A1 Location in patent:Page/Page column 74