
Phenol, 2-methoxy-4-(methylsulfonyl)- synthesis
- Product Name:Phenol, 2-methoxy-4-(methylsulfonyl)-
- CAS Number:1206968-73-1
- Molecular formula:C8H10O4S
- Molecular Weight:202.23
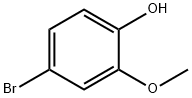
7368-78-7
189 suppliers
$24.00/1g
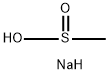
20277-69-4
265 suppliers
$10.00/1g

1206968-73-1
5 suppliers
inquiry
Yield:1206968-73-1 52%
Reaction Conditions:
copper(I) trifluoromethanesulfonate benzene;N,N`-dimethylethylenediamine in dimethyl sulfoxide at 130;
Steps:
26.1
[Example 26]tert-Butyl 4-[2-(4-methanesulfonyl-2-methoxyphenoxymethyl)pyridin-5-yl]piperidine-1-carboxylate (1) 4-Methanesulfonyl-2-methoxyphenol A mixture of 4-bromo-2-methoxyphenol (500 mg, 2.46 mmol), sodium methanesulfinate (1 g, 9.84 mmol), copper(I) trifluoromethanesulfonate benzene complex (124 mg, 0.25 mmol) and N,N'-dimethylethylenediamine (53μL, 0.49 mmol) in dimethyl sulfoxide (3 mL) was stirred overnight at 130°C. The mixture was allowed to cool to room temperature followed by the addition of ethyl acetate (8 mL) and water (8 mL). The resulting mixture was filtered through Celite pad and to the filtrate was added 2N hydrochloric acid and extracted with ethyl acetate. The organic layer was washed sequentially with water and brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/ethyl acetate=3/1→1/1) to give the title compound as a white crystal (258 mg, yield 52%).1H NMR(CDCl3, 400 MHz) : δ= 3.04(3H, s), 3.98(3H, s), 6.10(1H, s), 7.06(1H, d, J=8 Hz), 7.41(1H, d, J=2 Hz), 7.51(1H, dd, J=2 Hz, 8 Hz).
References:
EP2311822,2011,A1 Location in patent:Page/Page column 41