
Phenol, 3-bromo-4-ethyl- (9CI) synthesis
- Product Name:Phenol, 3-bromo-4-ethyl- (9CI)
- CAS Number:540495-28-1
- Molecular formula:C8H9BrO
- Molecular Weight:201.06
61791-99-9
71 suppliers
$12.00/100mg

540495-28-1
22 suppliers
$220.00/100mg
Yield:540495-28-1 96%
Reaction Conditions:
with triethylsilane;boron trifluoride diethyl etherate in dichloromethane at 0 - 30; for 12 h;
Steps:
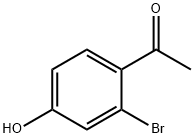
263.A Step A 3-bromo-4-ethylphenol.
To a solution of l-(2-bromo-4-hydroxy-phenyl)ethanone (2 g, 9.30 mmol, 1.0 eq) in DCM (40 mL) were added BFr'EtiO (2.64 g, 18.6 mmol, 2.30 mL, 2.0 eq) and triethylsilane (3.24 g, 27.9 mmol, 4.46 mL, 3.0 eq) at 0 °C. The reaction mixture was stirred at 30 °C for 12 hours. The reaction mixture was diluted with water (20 mL) and extracted with DCM (3 x 30 mL). The combined organic layers were washed with brine (30 mL), dried over NaiSOr, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (S1O2, petroleum ether/ethyl acetate= 100/1 to 5/1) to give the title compound (1.8 g, 96% yield). Yellow oil; NMR (400MHz, chloroform-d) d = 7.13 - 7.04 (m, 2H), 6.75 (dd, J= 2.8, 8.4 Hz, 1H), 2.69 (q, J= 7.6 Hz, 2H), 1.20 (t, J= 7.6 Hz, 3H).
References:
WO2021/41671,2021,A1 Location in patent:Paragraph 0980
52121-36-5
37 suppliers
$155.00/50mg

540495-28-1
22 suppliers
$220.00/100mg

100-12-9
102 suppliers
$35.00/25g

540495-28-1
22 suppliers
$220.00/100mg

52121-34-3
26 suppliers
$232.19/250mg

540495-28-1
22 suppliers
$220.00/100mg

64080-15-5
59 suppliers
$60.00/100mg

540495-28-1
22 suppliers
$220.00/100mg