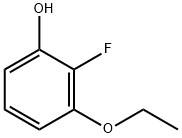
Phenol, 3-ethoxy-2-fluoro- synthesis
- Product Name:Phenol, 3-ethoxy-2-fluoro-
- CAS Number:1191998-59-0
- Molecular formula:C8H9FO2
- Molecular Weight:156.15

451-80-9
62 suppliers
$14.00/1g

1191998-59-0
6 suppliers
inquiry
Yield:1191998-59-0 22 g
Reaction Conditions:
Stage #1: 1-ethoxy-2-fluoro-benzenewith sec.-butyllithium in tetrahydrofuran;hexane;cyclohexene at -75 - -70; for 1 h;Inert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran at -75 - 25; for 3 h;Inert atmosphere;
Stage #3: with dihydrogen peroxide;acetic acid at 20 - 35; for 10.5 h;Inert atmosphere;
Steps:
1.2 Second Step
Under a nitrogen atmosphere, compound (s2) (20 g, 142.7 mmol) and THF (300 mL) were added into a reaction vessel, and the resulting mixture was cooled to -70° C. or lower. Then, sec-butyllithium (1.08 M; n-hexane/cyclohexane solution; 165 mL, 178.3 mmol) was added dropwise in the temperature range from -75° C. to -70° C., and the resulting mixture was further stirred for 1 hour. A THF (50 mL) solution of trimethyl borate (21.05 mL, 178.3 mmol) was added dropwise in the temperature range from -75° C. to -70° C., the resulting mixture was stirred for 3 hours while returning to 25° C. Thereto, acetic acid (12.25 mL, 214.0 mmol) was added dropwise in the temperature range of 20° C. to 25° C. After 30 minutes, hydrogen peroxide (30% aqueous solution, 32.3 g, 285.4 mmol) was added dropwise in the temperature range from 25° C. to 35° C., and the resulting mixture was further stirred for 10 hours. The reaction mixture was poured into water, and extracted with ethyl acetate. Combined organic layers were washed with water, an aqueous solution of sodium sulfite, water and saturated brine, and then dried over anhydrous sodium sulfate. The solution was concentrated under reduced pressure, and thus compound (s3) (22.0 g, 141.0 mol; 99 mol %) was obtained. Compound (s3) was used for the next reaction without further purification.
References:
US9523036,2016,B2 Location in patent:Page/Page column 41
367-12-4
417 suppliers
$5.00/10g

1191998-59-0
6 suppliers
inquiry