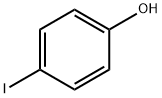
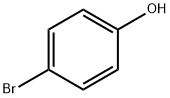
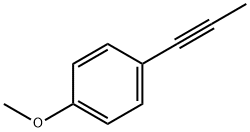
Phenol, 4-(1-propynyl)- (9CI) synthesis
- Product Name:Phenol, 4-(1-propynyl)- (9CI)
- CAS Number:170651-15-7
- Molecular formula:C9H8O
- Molecular Weight:132.16
Yield:170651-15-7 87%
Reaction Conditions:
Stage #1: p-Iodophenolwith bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;triethylamine in tetrahydrofuran at 0; for 0.166667 h;Inert atmosphere;Sonication;
Stage #2: prop-1-yne in tetrahydrofuran at -78 - 20;Inert atmosphere;Sonogashira Cross-Coupling;
Steps:
Sonogashira Coupling of Aryl Iodides with Propyne; General Procedure
General procedure: A flame dried flask was charged with THF (1.5 mL/mmol ArI), followed by CuI (10% equiv) and PdCl2(PPh3)2 (2.5% equiv) while argon was bubbled through. The solution was sonicated at 0 °C for the duration of all reagent additions except for propyne. The respective aryl iodide* (1 equiv) was dissolved in THF (1.5 mL/mmol ArI) and added dropwise to the solution, followed by dropwise addition of Et3N in THF (2-3 equiv/1.5 mL). Addition of reagents during sonication was done over the course of approximately 10 min. The solution was cooled to -78 °C and propyne in THF (2 equiv) was added. The solution was stirred and allowed to reach rt slowly overnight. The reactionwas worked up with sat. aq NH4Cl (1 × 7 mL/mmol ArI) and brine (3 × 5 mL). The organic fraction was poured directly from the separatory funnel onto a Celite pad topped with Na2SO4 and vacuum filtered. EtOAc was poured through to recover the remaining product. The solvent was removed in vacuo, affording a crude dark product with no aryl iodide present by TLC and 1H NMR analyses. The material was purified by either column chromatography or by vacuum distillation. The column was prepared by a hexane slurry of silica gel and loaded with crude material dissolved in a minimal amount of EtOAc. A gradient from 100% to 80% hexane with 20% EtOAc was performed, yielding the desired product. * Experimental scales from 1 mmol to 6 mmol of the aryl iodide were performed.
References:
Alterman, Joshua L.;Kraus, George A. [Synthesis,2021,vol. 54,# 3,art. no. SS-2021-M0500-PSP,p. 655 - 657]
2749-94-2
7 suppliers
inquiry

170651-15-7
2 suppliers
inquiry
214898-11-0
0 suppliers
inquiry

170651-15-7
2 suppliers
inquiry