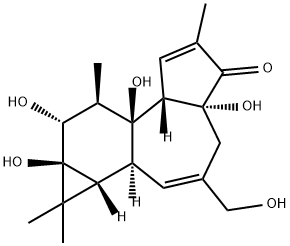
PHORBOL synthesis
- Product Name:PHORBOL
- CAS Number:17673-25-5
- Molecular formula:C20H28O6
- Molecular Weight:364.43
The optimized operation conditions of biphasic alcoholysis were a reaction time of 91 min, a temperature of 14°C, and a croton oil-methanol ratio of 1:30 (g:ml). The phorbol during the biphasic alcoholysis was 3.2-fold higher in content than that obtained in conventional monophasic alcoholysis. The optimized high-speed countercurrent chromatography method was using the ethyl acetate/n-butyl alcohol/water at 4.7:0.3:5 (v:v:v) with Na2SO4 at 0.36 g/10 ml as the solvent system, using the mobile phase flow rate of 2 ml/min, the revolution of 800 r/min, under which the retention of the stationary phase was achieved at 72.83%. The crystallized phorbol following high-speed countercurrent chromatography was obtained as high purity of 94%.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17673-25-5
109 suppliers
$59.00/1mg
Yield:-
Steps:
Multi-step reaction with 22 steps
1.1: bis(acetylacetonato)manganese(II); phenylsilane; oxygen / ethanol
2.1: triethylamine / dichloromethane / 0 °C
3.1: methyltrifluoromethyldioxirane / dichloromethane / 0 °C
4.1: zinc(II) iodide; magnesium iodide / diethyl ether
5.1: bis(acetylacetonato)manganese(II); phenylsilane; oxygen; triphenylphosphine / ethanol
6.1: ruthenium trichloride; sodium bromate; sodium hydrogencarbonate / ethyl acetate; acetonitrile; water
7.1: dmap / dichloromethane / 0 °C
8.1: dmap / dichloromethane / 0 °C
9.1: triethylamine / N,N-dimethyl-formamide / 60 °C
10.1: methanol / Reflux
11.1: acetic acid; sodium cyanoborohydride / methanol / Reflux
12.1: chromium(VI) oxide; 3,5-dimethyl-1H-pyrazole / dichloromethane / 0 - 20 °C
13.1: trimethylsilylazide / 1,2-dichloro-ethane / 70 °C
13.2: 70 °C
14.1: bis(benzonitrile)palladium(II) dichloride; triphenyl-arsane; copper(l) iodide / 1-methyl-pyrrolidin-2-one / 80 °C
15.1: pyridine hydrogenfluoride / tetrahydrofuran / 0 °C
16.1: Martins sulfurane / 1,2-dichloro-ethane / 60 °C
17.1: selenium(IV) oxide / benzene / 80 °C
18.1: sodium tetrahydroborate / methanol / -40 °C
19.1: dmap / dichloromethane
20.1: sodium tris(acetoxy)borohydride / benzene / Reflux
21.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 0 °C
22.1: barium(II) hydroxide / methanol
References:
Kawamura, Shuhei;Chu, Hang;Felding, Jakob;Baran, Phil S. [Nature,2016,vol. 532,# 7597,p. 90 - 93]