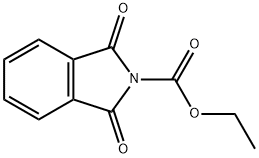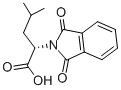
PHT-LEU-OH synthesis
- Product Name:PHT-LEU-OH
- CAS Number:2419-38-7
- Molecular formula:C14H15NO4
- Molecular Weight:261.27
Yield:2419-38-7 100%
Reaction Conditions:
with sodium carbonate in water at 20; for 2 h;
Steps:
2
L-leucine (5.12 g, 39.1 mmol) and Na2CO3 (4.14 g, 39.1 mmol) were dissolved in 40 mL distilled H2O. The solution was added 7V-carbethoxy-phathalimide (8.55 g, 39.1 mmol) and then stirred at room temperature for 2 h. The resultant clear solution was acidified using 6 N HCl to pH=0 and then extracted with hexanes (3* 100 mL). The combined organic layers were dried in vacuo. Column chromatography on silica gel was applied eluting with hexanes/acetone (3:1) to get JV-phthalimide-L-leucine (1) (10.2 g, 39.1 mmol, quantitative) as colorless oil. 1H NMR (400 MHz, CDCl3) £0.92 (d, J=6.70 Hz, 3 H), 0.94 (d, J=6.70 Hz, 3 H), 1.37 - 1.60 (m, 1 H), 1.95 (ddd, J=14.31, 10.05, 4.26 Hz, 1 H), 2.36 (ddd, 7=14.31, 10.05, 4.26 Hz, 1 H), 4.99 (dd, J=I 1.57, 4.26 Hz, 1 H), 7.73 (dd, 7=5.48, 3.05 Hz, 2 H), 7.85 (dd, 7=5.48, 3.05 Hz, 2 H), 11.32 (br. s., 1 H); 13C NMR (75 MHz, CDCl3) δ: 21.2, 23.3, 25.3, 37.2, 50.6, 123.8, 131.9, 134.4, 167.9, 176.0
References:
WO2010/20055,2010,A1 Location in patent:Page/Page column 68
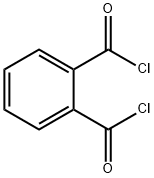
88-95-9
163 suppliers
$10.00/25g

2419-38-7
53 suppliers
$11.19/1G
![Benzoic acid, 2-[[[(1S)-1-carboxy-3-methylbutyl]amino]carbonyl]-](/CAS/20180529/GIF/6707-69-3.gif)
6707-69-3
0 suppliers
inquiry

61-90-5
780 suppliers
$10.00/25G
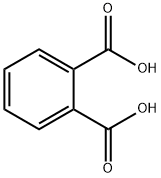
88-99-3
440 suppliers
$15.00/50g

2419-38-7
53 suppliers
$11.19/1G