
Phthalazine, 1,4-dichloro-6-(trifluoromethyl)- synthesis
- Product Name:Phthalazine, 1,4-dichloro-6-(trifluoromethyl)-
- CAS Number:26238-16-4
- Molecular formula:C9H3Cl2F3N2
- Molecular Weight:267.03

26238-15-3
0 suppliers
inquiry

26238-16-4
1 suppliers
inquiry
Yield:26238-16-4 91%
Reaction Conditions:
with pyridine;trichlorophosphate for 1 h;Reflux;
Steps:
5.1.21. 1,4-Dichloro-6-(trifluoromethyl)phthalazine (12b)
A mixture of 11b (176 mg, 0.76 mmol, 1.0 equiv.) and pyridine(123.6 mL, 1.53 mmol, 2.0 equiv.) in POCl3 (2.5 mL) was heated atreflux for 1 h. After cooling, the reaction mixture was concentratedunder vacuum. The residue was dissolved in cooled DCM andpoured slowly into ice-cold wate. The organic phase was separatedand the aqueous phase was extracted twice with DCM. The combinedorganic layers were washed with a saturated solution ofNaHCO3 and brine, dried over Na2SO4, filtered, concentrated undervacuum and purified by silica gel column chromatography (heptanes:EtOAc 7:3 to yield 12b as a white solid (186 mg, 0.70 mmol,91%). 1H NMR (400 MHz, CDCl3) δ ppm 8.26 (dd, 1H, J 1.8 Hz,J 8.7 Hz), 8.49 (dt, 1H, J 0.8 Hz, J 8.7 Hz), 8.61 (t, 1H, J 0.8 Hz);19F NMR (376 MHz, CDCl3) d ppm 63.1; 13C NMR (101 MHz, CDCl3) δ ppm 121.5, 123.9 (q, J 4.4 Hz), 124.2, 127.2, 127.7, 128.9, 130.6 (q,J 2.9 Hz), 136.5, 155.2 (d, J 34.5 Hz).
References:
Bollenbach, Maud;Lugnier, Claire;Kremer, Mélanie;Salvat, Eric;Megat, Salim;Bihel, Frédéric;Bourguignon, Jean-Jacques;Barrot, Michel;Schmitt, Martine [European Journal of Medicinal Chemistry,2019,vol. 177,p. 269 - 290]

1997-41-7
19 suppliers
inquiry

26238-16-4
1 suppliers
inquiry
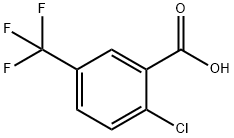
657-06-7
189 suppliers
$7.00/1g

26238-16-4
1 suppliers
inquiry
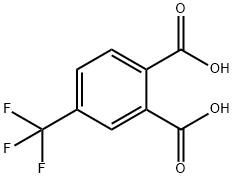
835-58-5
80 suppliers
$35.00/250mg

26238-16-4
1 suppliers
inquiry