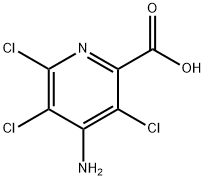
Picloram synthesis
- Product Name:Picloram
- CAS Number:1918-02-1
- Molecular formula:C6H3Cl3N2O2
- Molecular Weight:241.46

35592-96-2
9 suppliers
$28.60/100mg

1918-02-1
277 suppliers
$14.14/1gm:
Yield:1918-02-1 95.5%
Reaction Conditions:
with ammonia in methanol;lithium hydroxide monohydrate at 120 - 130; for 8 h;Autoclave;Large scale;
Steps:
1.1-1.2; 2.1-2.2; 1 1) Ammonolysis reaction
Into a 2L autoclave, add 281 g of methyl 3,4,5,6-tetrachloropicolinate (97.9%), 1200 g of methanol (1500 ml), and 50 ml of water. After the addition is completed, the autoclave is closed, and the temperature is kept at 20 under stirring. 170 grams of ammonia gas was slowly introduced into it within .The autoclave was heated to 120-130° C., and the reaction was maintained for 8 hours. After sampling, the HPLC tracked that the residual 3,4,5,6-tetrachloropicolinic acid (intermediate product) was no higher than 1% to complete the reaction.2) Post-processingAfter cooling the material, transfer it to a distillation flask to remove the solvent at normal pressure, the liquid temperature is 80-85 °C, and receive about 1270-1300 grams of methanol-ammonia solution, add 200 ml of water, continue to distill until the liquid temperature is 98-100 °C, stop Distilled.Stir to cool down and add 100 grams of industrial hydrochloric acid (36%) dropwise to determine whether pH=2 is reached, if not, continue to add a small amount of hydrochloric acid to pH=1-2, and end the dropwise addition.The material was heated to 50-60°C, matured and grown for 2 hours, cooled to below 30°C, filtered and rinsed with 100ML water, and dried to obtain 234.9 g of finished product of amlidine. The purity was 98.2% as determined by high performance liquid chromatography, and the yield was 95.5%. .
References:
CN114702440,2022,A Location in patent:Paragraph 0046-0053

1336-21-6
711 suppliers
$14.56/250ML
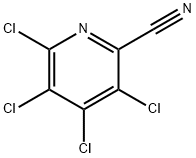
17824-83-8
68 suppliers
$66.00/5mg

1918-02-1
277 suppliers
$14.14/1gm:
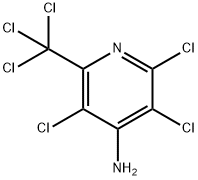
5005-62-9
9 suppliers
inquiry

28443-69-8
57 suppliers
$45.00/10mg

1918-02-1
277 suppliers
$14.14/1gm: