Piperazine, 1-(2,6-dimethylphenyl)-4-methyl- synthesis
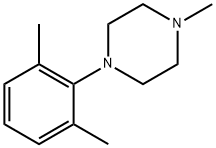
- Product Name:Piperazine, 1-(2,6-dimethylphenyl)-4-methyl-
- CAS Number:1438279-35-6
- Molecular formula:C13H20N2
- Molecular Weight:204.31
Yield: 95%
Reaction Conditions:
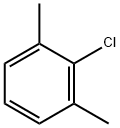
with C34H39N3O4Pd;sodium t-butanolate in 1,4-dioxane at 100; for 0.5 h;Schlenk technique;Inert atmosphere;Buchwald-Hartwig Coupling;
Steps:
4.4 General procedure for the Buchwald-Hartwig amination reactions
General procedure: In air, to a Schlenk tube that closed with a screw cap fitted with a septum and was equipped with a magnetic stir bar were added in turn complex 3 (0.01 mmol, 7 mg), and NaOBut (1.5 mmol, 144 mg). The tube was then caped with a rubber septum and evacuated and backfilled with argon. This sequence was repeated three times. Aryl chloride (1.0 mmol) and amine (1.1 mmol) was injected through the septum by syringe, followed by addition of dry 1,4-dioxane (10 mL). The mixture was then stirred at a pre-heated oil bath (100 °C) for the indicated time. The reaction mixture was cooled to room temperature and CH2Cl2 (25 mL) added. After filtration via a short pad of celite, the filtrate was condensed under vacuum and the residue was purified by flash chromatography on silica gel to provide the desired coupling products. Yields of isolated products were reported in Tables 1-3.
References:
Li, Yan-Jing;Zhang, Jin-Ling;Li, Xiao-Jian;Geng, Yu;Xu, Xiao-Hua;Jin, Zhong [Journal of Organometallic Chemistry,2013,vol. 737,p. 12 - 20]